Abstract
Because germ line genetic testing is increasingly integrated into the clinical care of patients with hematologic malignancies, it is important for hematologists to effectively communicate with patients and their families about the genetic testing process and to relay the results in a concise and understandable manner. Effective communication facilitates trust between patients and providers and allows patients to feel empowered to ask questions and actively participate in their health care. Especially for inherited conditions, the patient’s understanding of germ line genetic information is critical because it enables them to share this information with relatives who are at risk, thereby promoting cascade testing and providing potentially life-saving information to family members who may be similarly affected. Accordingly, a hematologist’s skills in understanding the importance and implications of germ line genetic information and the ability to convey this information in patient-friendly language is a critical first step and can have a far-reaching impact. In this article, we outline a straightforward approach to discussing genetic information and provide the reader with practical tips that can be used when consenting patients to germ line genetic testing and disclosing subsequent test results. We also review special considerations and ethical concerns arising when offering genetic evaluation and germ line testing to patients and related donors for allogeneic hematopoietic stem cell transplantation.
Introduction
Advances in genetic testing technologies have led to the development of assays that are capable of examining large numbers of genes and affecting the medical management of patients and their family members.1 As a result, it is now possible to comprehensively query tumor and germ line genomes, leading to the discovery of targetable lesions in tumors and predisposing variants in the germ line.2 Genetic testing on the blood or bone marrow (BM) of patients with a hematologic malignancy can aid in diagnosis, risk stratification, and management decisions. Accordingly, such testing has become a standard practice.3 Although this testing is targeted to the malignant cells, it can also reveal information that raises suspicion of an underlying predisposition (Table 1), and the hematologist must be prepared to handle expected as well as unexpected results.2
An ever-increasing array of genetic conditions associated with familial leukemia has been identified.4-8 With expanding knowledge of these conditions, the importance of germ line genetic testing for patients undergoing curative or pre-emptive allogeneic hematopoietic stem cell transplantation (allo-HSCT) grows. Genetic testing for these conditions can affect decisions surrounding allo-HSCT by identifying family members who have an underlying predisposition and should thus be avoided as potential donors. Testing can also inform approaches to conditioning regimens in patients with inherited BM failure syndromes for whom standard regimens may result in excess toxicity. Accordingly, hematologists should be aware of the unique challenges and ethical issues that can arise in the setting of genetic evaluation of related stem cell donors.9
The cost of testing is declining and the speed is increasing, allowing for more accessible genetic testing and more frequent opportunities to use germ line results to actively guide clinical care. Nevertheless, as sequencing technologies become more comprehensive and of higher resolution, the amount of data generated expands exponentially. Further complicating matters, underrepresentation of certain racial and ethnic groups in genomic databases is leading to increased likelihood of identifying variants of uncertain significance (VUSs) among individuals of non-European ancestry. Consequently, there are increasing germ line variants being reported that demand informed interpretation and decision-making by the ordering clinician. As might be expected, this explosion of genetic information allows for unique opportunities to optimize patient care, but, at the same time, it brings with it many challenges related to the communication of genetic information.
Given the importance and complexity of germ line genetic information, it is essential that hematologists understand the benefits and limitations of the tests they offer. Furthermore, they should inform patients in a clear and meaningful manner about the testing process, types of test results, and, importantly, the potential implications of these results (in terms of health risks and clinical care). Although several excellent commentaries have been written on the topics of germ line genetic testing and informed consent in hematology,10,11 none, to our knowledge, provide basic tips and practical language to facilitate these important conversations.
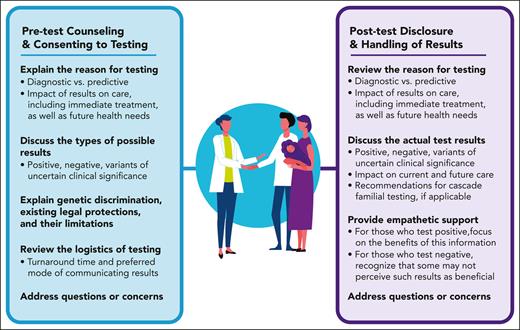
This article will use a case-based approach to highlight salient points surrounding:
pretest counseling and consenting for germ line testing,
posttest disclosure and handling of test results, and
germ line testing in the setting of allo-HSCT.
Case 1: pretest counseling and consenting for germ line testing
A 13-year-old girl presented with fatigue, pallor, and malaise, and a complete blood count that revealed pancytopenia. Evaluation of the blood smear revealed blasts, whereas flow cytometric evaluation of the BM confirmed B-cell acute lymphoblastic leukemia. Cytogenetic analysis demonstrated a modal chromosome count of 39 chromosomes consistent with low hypodiploidy. The family history was significant for a sibling diagnosed with adrenocortical carcinoma who died from therapy-associated myelodysplastic syndrome. Given the history of 2 children with cancers concerning for Li-Fraumeni syndrome (LFS), the family was counseled regarding this possibility and utility of germ line genetic testing. After taking several days to consider whether they wanted to learn this information, the parents and adolescent girl agreed to undergo testing of her skin fibroblasts. This revealed a pathogenic variant in TP53, confirming the diagnosis of LFS.
Key components of pretest counseling
Case 1 presents numerous factors that a hematologist must consider before proceeding with germ line testing, including the timing and content of consent conversations and, importantly, how the information can best be presented to patients and their families (hereafter denoted as patients).12 In terms of timing, patients vary in their preferences regarding these conversations. For most, the period surrounding a suspected diagnosis of a hematologic malignancy is stressful, marked by anxiety, uncertainty, and preoccupation with completing any needed procedures. Therefore, it may be difficult for patients to concentrate on discussions about germ line testing.13
When performing genetic testing on the blood or BM of patients with hematologic malignancies, it is important to explain that the goal of testing is to assist with diagnosis and therapy but not to identify an underlying genetic condition. Nevertheless, information from testing leukemic cells can raise suspicion of an existing genetic predisposition. Patients should be aware that follow-up testing using a skin biopsy or other germ line sample might be needed. This can be communicated by explaining that testing a blood or BM sample that contains leukemia cells may reveal mutations that arise within these cells. To determine whether one of these mutations is associated with an underlying predisposition, it is helpful to test skin fibroblasts or possibly hair bulbs, which contain genetic changes that are present since birth.
Many patients will agree to germ line analysis without understanding the implications of testing. Therefore, it is important to be mindful of each patient’s specific clinical context and provide flexibility in the timing of conversations about germ line genetic testing, particularly if the results are not going to inform immediate treatment decisions.14,15 If possible, it is advisable to involve a certified genetic counselor or other genetics provider who can carry out or assist with discussions surrounding germ line testing and address patient questions and concerns.16
When initiating conversations about genetics, it is helpful to establish an agreed upon agenda and meet patients at their level of comprehension. Before beginning, ask patients whether they understand the purpose of the visit and inquire about their baseline level of genetic knowledge. Most patients have limited knowledge of genetics, particularly when it comes to the differences between tumor and germ line testing.13,17 Therefore, encourage patients to ask questions. Recognizing that many patients may not remember much of what is discussed, inform them that you are willing to meet with them again to review the information that was presented. It is our experience and that of others, that having more than one conversation improves the patient’s understanding of genetics.15,17 Indeed, in case 1, the patient and parents met with the provider shortly after diagnosis but were not ready to undergo testing. A follow-up visit was scheduled during which the provider reviewed key concepts and answered questions, providing the family with the information needed to provide informed consent. Not all hematologists will be able to sustain a model of care that allows for multiple visits before genetic testing. Therefore, providers should be aware of alternative genetic counseling resources (Tables 2 and 3).
In terms of the content of the consent conversation, the first focus is the “why” of testing. In case 1, germ line genetic testing was performed for diagnostic purposes (to determine whether the patient developed the disease because of an underlying genetic predisposition). This differs from predictive testing, in which individuals who do not manifest with disease are offered testing to determine whether they are at risk for developing the disease. For some patients, identifying an underlying predisposition can provide emotional benefit by explaining why a cancer developed and allowing them to prepare for the possibility of a future cancer or other health concerns.18 Many individuals undergo germ line testing for hereditary cancer to pursue cancer screening or preventive measures.19 In case 1, the diagnosis of LFS confers an increased risk for future cancers,20,21 with effective screening now available.22,23 In some cases, the germ line information can direct cancer treatment, such as avoidance of radiation therapy or specific genotoxic agents, or pursuit of allo-HSCT. In addition to their own care, many patients are interested in germ line information because it will enable genetic counseling and testing of other family members, who can then speak with their providers about individualizing their own health care.18 Providing clear information about how germ line information might be used to guide the patient’s care, as well as the care of any relatives, is a key part of enabling a patient to make informed decisions about testing.
It is important to consider the age of the patient when discussing germ line genetic testing, because the reasons to pursue such testing may change over the life course of an individual. A child or teenager providing assent for testing may be focused on the hematologic malignancy and its treatment but not on the future. Children and adolescents should be asked what level of information they prefer and allowed to ask questions and share concerns. In contrast, a young adult may be considering testing for family planning, whereas an older adult may prefer testing because they are worried about risks for their children and relatives. Tailoring the discussion based on the patient’s age and life circumstances is key to obtaining quality informed consent.
Although many patients with leukemia endorse high interest in genetic testing, few barriers, and relatively low distress,24 potential concerns and barriers do exist. In our survey of parents whose children underwent germ line testing of a large panel of cancer predisposition genes, uncovering a pathogenic variant was the most frequently endorsed risk of testing.18 Thus, for some patients, germ line information can bring with it significant emotional and/or psychological burdens, including increased worry and uncertainty about the future. Patients may also be concerned about loss of privacy and discrimination by insurers or employers.25 For patients in the United States, concerns for genetic discrimination can be lessened by discussing the Genetic Information Nondiscrimination Act (GINA), a law passed in 2008 that protects individuals undergoing germ line genetic testing from certain forms of genetic discrimination.26 For example, it is illegal for health insurance providers to make decisions about coverage or premium rates and for employers with ≥15 employees to make hiring, firing, or promotion decisions based on genetic test results. GINA does not protect against life, long-term care, or disability insurance companies from using genetic information when making coverage or premium decisions. Insurance companies can ask about germ line test results, and it is the responsibility of the individual seeking coverage to provide this information. In addition, legislation varies in different countries. Ultimately, it is important that hematologists appreciate that each patient perceives genetic risk differently and understand that what some patients find beneficial, others may see as a challenge.
An integral component of the pretest discussion is a description of the possible types of germ line test results, including positive (one or more disease-associated variants were identified; these are also known as pathogenic or likely pathogenic variants), negative (no reportable variants identified) and VUSs (one or more variants identified, but there is insufficient evidence to determine whether these variants are associated with disease). The hematologist should also mention the possibility of uncovering unexpected or incidental variants (variants not related to the purpose of testing). Making families aware that results are not always straight forward and preparing them for the possibility of uncertainty allows for truly informed consent and can make the disclosure of germ line results less surprising or stressful.
Once consent conversations are complete, the hematologist should ask patients whether they want results returned in person, via phone, or via telehealth. Finally, it is important to recognize that not all patients choose to complete germ line testing. In recent studies, >12% of patients declined such testing for themselves or their children.25,27 Reasons for declining included feeling overwhelmed because of a recent diagnosis, concerns about insurance discrimination, not wanting to learn results, and perceived lack of benefit. In situations in which patients decline germ line testing, the hematologist should honor the patient’s decision. Although a patient may decline such testing in the throes of a new diagnosis, their preferences might change over time. Therefore, the hematologist should encourage patients to reach out in the future should they wish to revisit testing options.
Case 2: posttest disclosure and handling of test results
A 5-year-old boy presented to the emergency department with fever and fatigue and was found to have peripheral blasts. Flow cytometry results were consistent with an acute myeloid leukemia (AML) diagnosis. Diagnostic workup included a hematologic somatic malignancy genetic panel performed on a BM aspirate, which revealed a pathogenic variant in the BRCA2 gene at a 42% variant allele frequency (VAF). Because somatic variants in BRCA2 are highly unusual in acute leukemia, suspicion was raised that this variant was germ line related.
Key components of posttest counseling
Case 2 demonstrates how tumor testing can uncover potential germ line results, including those that are unexpected. To the best of our knowledge at present, heterozygous BRCA2 variants are not strongly linked to risks of hematologic malignancy.28 This scenario is becoming increasingly common, and it is crucial for providers to become familiar with interpreting the results of tumor testing and understanding the findings that raise suspicion of being of germ line origin. This information then needs to be communicated with the family so that confirmatory germ line testing can be coordinated for the patient and possibly cascade to testing among the family.
Although the patient in case 2 had no overt physical findings, the provider should consider the possibility of occult Fanconi anemia, which is associated with an increased risk for AML and solid tumors and can be caused by biallelic germ line variants in BRCA2.29 However, a second hit in BRCA2 was not identified through the testing of this child’s leukemia. Hematologists should understand that malignancy gene panels do not always interrogate the entire coding regions of genes, and they may not detect intronic or copy number variants. Therefore, when there is strong clinical suspicion of a predisposition, tumor gene panels should not be used as a substitute for germ line testing. Because of the increased toxicities associated with DNA-damaging chemotherapies for patients with Fanconi anemia, ruling out this diagnosis was critical for the patient in case 2.
Before disclosing genetic results (somatic or germ line), it is helpful to ask patients what they remember about any prior discussions of genetic testing, such as the discussion that occurred when they provided consent. During the result disclosure, the family in case 2 was not ready to hear the details, so the provider informed them that a genetic variant, also referred to as a mutation, was identified in the leukemia cells and that it was necessary to perform a second test to determine whether the result was important for the child’s care. The hematologist obtained consent to send the sample for chromosome breakage testing, which returned with normal results, significantly reducing the likelihood that the patient had Fanconi anemia. At a follow-up visit, the family was prepared to move forward with discussions of germ line testing. As for case 1, it can be helpful to start the conversation by reviewing at a high level that germ line genetic information is helpful because it can enable personalized management for the patient and family. This can be followed up with a deeper discussion of future cancer risks and available surveillance or risk-reducing recommendations.
For this family, the provider then explained that it is possible to determine whether the BRCA2 variant was germ line–related by testing skin fibroblasts from the patient or testing for and identifying the variant in a close family member (usually a parent). Examples of phrases to be used to explain the differences between germ line and somatic variants are detailed in Table 2. The provider focused on the benefits and emphasized that surveillance and cancer risk reducing options can be life-saving. The hematologist reinforced that no immediate changes to care were needed for the patient based on a heterozygous BRCA2 variant but informed the family that it will be important for the patient to consult a genetic counselor when he is a young adult to review the surveillance, prevention, and treatment options for BRCA2-associated hereditary breast and ovarian cancer syndrome. Because genetic information can affect the health of relatives, it is important for the hematologist to discuss whether a patient plans to communicate this information to others in the family. We find that providing the patient with a letter explaining the genetic result along with a list of resources to facilitate germ line testing helps patients carry out these important but difficult conversations. A list of pertinent resources for providers and patients is included in Table 3.
During result disclosure conversations, the hematologist should emphasize that having a mutation does not mean that the tested individual will definitively develop a cancer. Rather, these individuals have an increased risk compared with others in the population. Normalizing these risks can ease feelings of guilt. For many families, access to educational materials facilitates understanding. Therefore, it is helpful to offer information about the condition and patient support or advocacy groups. Finally, remind patients that it is normal to feel overwhelmed when learning about inherited genetic risk, and give them time to process and have follow-up meetings. In these conversations, the roles of the hematologist are to focus on disclosure of results and provide basic genetic information in an understandable manner. The hematologist does not need to be an expert on the genetic condition. However, he or she should understand when and how to refer patients to a genetics provider (or other specialist) for further discussion or evaluation.
Although case 2 focuses on discussing an unexpected positive result with a patient, it is equally important for providers to understand how to discuss VUSs and negative results. In general, medical management, such as cancer treatment, surveillance, or prevention, is only affected by positive results. In contrast, management is not generally altered for individuals with VUSs, nor are other family members tested for these VUSs. In some situations, providers may choose to act upon a VUS, such as a novel or rare variant predicted to affect a functional domain in a protein linked to a patient’s disease phenotype. In the case of a suspicious VUS, the concerning features about the variant are usually detailed and conveyed in the genetic report.
For patients with a negative result, it is important to explain that the result is reassuring; however, it does not mean that there are no genetic risks present. Rather, no alterations were identified in the genes analyzed via the testing ordered. Current understanding of genes and novel associations with diseases are evolving. Therefore, with expanded testing in the future, an answer could be provided even if none is identified on the present testing. Understanding and conveying the meaning of a negative result is also relevant when there is a strong family history of cancer that remains unexplained and family members are being considered as stem cell donors. In certain circumstances, an unrelated individual may be selected as a donor instead of a matched family member. This can occur if there are concerns that a predisposition is present in the family member despite the absence of an identifiable germ line mutation. In rare cases, it may be reasonable to use such a family member as long as he or she has undergone a thorough physical and hematologic assessment and no abnormalities are identified. It is critical for the cancer genetics and transplant teams to partner with the patient in making these decisions based on information specific to the patient. Table 4 reviews common types of genetic results and provides information on how to explain them and when to coordinate cascade testing.
Case 3: germ line testing in the setting of allo-HSCT
A 66-year-old man with normal karyotype AML underwent matched sibling donor allo-HSCT. Two years later, upon routine examination of the BM, the conventional karyotype analysis demonstrated a deletion of 7q in the setting of full donor chimerism. He had a normal complete blood count result, with the exception of macrocytosis. The BM showed mild trilineage dysplasia. His brother and uncle had both died of AML several years before his presentation. Further genetic testing of the post allo-HSCT BM aspirate revealed a likely pathogenic DDX41 variant with a VAF of 51%. Given the full donor chimerism, the DDX41 variant was suspected to be of donor origin.
DNA from the BM at his AML diagnosis was examined for DDX41 variants. This assessment again demonstrated the likely pathogenic DDX41 variant along with a somatic second hit to the other DDX41 allele, suggesting that his AML likely occurred as the result of a DDX41 germ line predisposition. Indeed, the DDX41 variant with a VAF of 51% was confirmed to be of germ line origin via an examination of hair follicle DNA and was subsequently detected in the blood sample of his healthy brother, the stem cell donor. Together, these results indicate that the patient developed donor-derived deletion 7q myelodysplastic syndrome (MDS) after receiving stem cells from his genetically affected but clinically asymptomatic brother. After genetic testing of the extended kindred, it was shown that the patient’s deceased brother and uncle were obligate carriers of the DDX41 germ line variant, consistent with their AML also occurring because of DDX41 predisposition to myeloid malignancy.
Key components of genetic testing in the setting of allo-HSCT
Since the first report in 2015 of pathogenic germ line variants in DDX41 conferring a predisposition to myeloid neoplasm,30 there has been a rapid recognition of hundreds of cases, leading to DDX41 being the most commonly reported MDS or AML predisposition gene in adults, underlying >5% of adult AML cases.31 Median age of disease onset is later in life, in the seventh decade.32 Disease penetrance appears to be modest, reaching ∼40% by the age of 90 years.33 Less than 30% of patients report a family history of hematologic malignancy,34 reflective of both the later age of disease onset and reduced penetrance. Inadvertent use of genetically affected stem cells from a familial donor may result in adverse transplant outcomes including donor-derived leukemia.35 The Worldwide Network for Blood and Marrow Transplantation has recently recommended avoidance of stem cell donation by familial donors who carry a known hereditary hematologic malignancy predisposition gene (regardless of donor age).36 Given the lack of a positive family cancer history in many DDX41 cases (as well as for patients with other forms of hereditary leukemia) and resultant lack of clinical suspicion of germ line predisposition, there is now rationale for consideration of routine testing for germ line predisposition in all patients undergoing work up for familial allograft for MDS or AML.
The germ line genetic landscape of hematologic disorders is rapidly advancing, bringing with it associated changes in clinical practice. Had the 66-year-old man in case 3 presented today, it is likely that he would have had the somatic and germ line DDX41 variants detected in the diagnostic stage of his leukemia workup, and an alternative stem cell donor would have been sought. Upon determination of a DDX41 germ line predisposition in a patient in the setting of planned allo-HSCT, providers should seek to rapidly counsel and test any family members who might genetically be at risk and are identified as potential donors for the variant present in the patient with the hematologic malignancy. Because this testing is targeted to a single variant, the hematologist can remind families that it is more rapid and less costly than the initial testing that was pursued for the patient. Furthermore, certain laboratories provide targeted testing of familial variants free of charge as long as such testing is ordered within a certain timeframe of the proband’s initial test. Nevertheless, it is important to respect the wishes of the family members and be aware that they may not be interested in receiving genetic information. Donors should have separate appointments from those of the family member undergoing the transplant so that they can discuss any concerns about genetic testing and make an independent decision about whether to proceed. An additional challenge centers on the ability to obtain rapid testing for potential donors because transplant decisions are often time sensitive. However, some genetics clinics and genetic counseling services can offer appointments within a week for urgent patients and work with the patient to obtain insurance coverage and/or reduce financial barriers to testing.
In the event of a transplant occurring in the setting of unrecognized germ line disease, one must consider how to deliver the information to the affected family members. For case 3, the suspicion of germ line disease was initially conveyed to the patient, and confirmatory tests were carried out swiftly thereafter. A separate appointment was made for the donor and his physician, and the timing of this meeting occurred soon after the proband’s appointment, because the anxiety-inducing nature of the discussions between the family members meant that it was important for the donor brother to have the opportunity to discuss the concerns with a medical professional in a timely manner. In due course, genetic appointments with counseling and testing of other family members who might be at risk were facilitated. Family members who harbored the familial variant were referred for hematologic evaluation and encouraged to have ongoing follow-up. It is acknowledged, however, that best management and surveillance of asymptomatic family members is still being determined for individuals with DDX41 germ line predisposition.
In the unrelated donor setting, pathways to communicating unexpected results related to pathogenic variants of donor germ line origin are not well-defined. The consent process for unrelated stem cell donation varies in different countries. Many countries do not have a standard procedure to provide genetic information gleaned from testing of the donated product back to the donor. Furthermore, many donors have not been consented for the return of this information. Ideally, future consent procedures will incorporate questions as to whether a stem cell donor prefers to be recontacted and informed of future germ line findings. Limiting posttransplant testing of patients to detect the presence of specific malignancy-associated somatic variants could be considered but requires coordination across clinical laboratories. With the increasing use and breadth of genetic panels for the evaluation of a possible relapse of a hematologic malignancy in the postallo-HSCT setting, formal guidelines will be needed to inform when and how to apply such tests and how to manage unexpected results that may have implications for both familial and unrelated donors.
Conclusions
Genetic testing is a key part of the diagnosis, risk prediction, and care for patients with malignant hematologic conditions. When possible, hematologists should work closely with genetic counselors or other genetics providers to facilitate consent for germ line testing and disclosure of results, covering key topics before and after testing (Table 5). This article and the resources provided herein aim to equip hematologists with the skills needed to communicate genetic information to patients, thereby improving patient understanding of results and follow-up care.
Acknowledgments
The authors thank Briana Williams (the graphic designer of Scientific Communications under Strategic, Communications, Education, and Outreach) at St. Jude Children’s Research Hospital for creating the graphical abstract that accompanies this article.
This work was funded in part by the American Lebanese Syrian Associated Charities (K.E.N.).
Authorship
Contribution: K.V.H., L.C.F., and K.E.N. contributed to conceptualizing, writing, and editing this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kim E. Nichols, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS1170, Memphis, TN 38105; e-mail: kim.nichols@stjude.org.