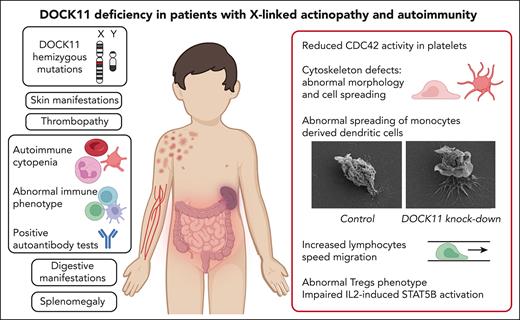
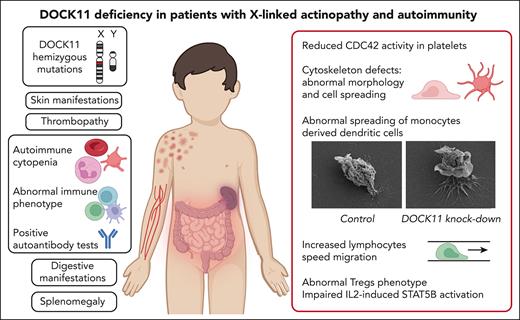
Key Points
DOCK11 deficiency is a new X-linked immune-related actinopathy.
DOCK11 deficiency leads to impaired CDC42 activity, abnormal actin cytoskeleton remodeling, and immune dysregulation.
Abstract
Dedicator of cytokinesis (DOCK) proteins play a central role in actin cytoskeleton regulation. This is highlighted by the DOCK2 and DOCK8 deficiencies leading to actinopathies and immune deficiencies. DOCK8 and DOCK11 activate CDC42, a Rho–guanosine triphosphate hydrolases involved in actin cytoskeleton dynamics, among many cellular functions. The role of DOCK11 in human immune disease has been long suspected but, to the best of our knowledge, has never been described to date. We studied 8 male patients, from 7 unrelated families, with hemizygous DOCK11 missense variants leading to reduced DOCK11 expression. The patients were presenting with early-onset autoimmunity, including cytopenia, systemic lupus erythematosus, skin, and digestive manifestations. Patients' platelets exhibited abnormal ultrastructural morphology and spreading as well as impaired CDC42 activity. In vitro activated T cells and B-lymphoblastoid cell lines from patients exhibited aberrant protrusions and abnormal migration speed in confined channels concomitant with altered actin polymerization during migration. Knock down of DOCK11 recapitulated these abnormal cellular phenotypes in monocytes-derived dendritic cells and primary activated T cells from healthy controls. Lastly, in line with the patients’ autoimmune manifestations, we also observed abnormal regulatory T-cell (Treg) phenotype with profoundly reduced FOXP3 and IKZF2 expression. Moreover, we found reduced T-cell proliferation and impaired STAT5B phosphorylation upon interleukin-2 stimulation of the patients’ lymphocytes. In conclusion, DOCK11 deficiency is a new X-linked immune-related actinopathy leading to impaired CDC42 activity and STAT5 activation, and is associated with abnormal actin cytoskeleton remodeling as well as Treg phenotype, culminating in immune dysregulation and severe early-onset autoimmunity.
Introduction
Immune-related actinopathies are inborn errors of immunity owing to gene defects affecting actin cytoskeleton remodeling. More than 20 entities are described so far.1 Actin cytoskeleton remodeling is a dynamic and tightly regulated process crucial for cell shape modifications, cell adhesion, motility, and other main cellular functions.2 Rho–guanosine triphosphate hydrolases (GTPases), RAS-related guanosine triphosphate-binding proteins, such as RAC, CDC42, and RHO, are key players in the intracellular actin reorganization.3,4 The phenotypic spectrum of RAC2 mutations ranges from granulocytes deficiency (loss-of-function mutations) to severe combined immunodeficiency with bone marrow hypoplasia (gain-of-function mutations).5 De novo mutations in CDC42 were recently found in patients presenting with Takenouchi-Kosaki syndrome, with associating features such as macrothrombocytopenia and developmental abnormalities; or with neonatal cytopenia with dyshematopoiesis, autoinflammation, rash, and hemophagocytic lymphohistiocytosis, termed NOCARH syndrome.6 Rho-GTPases are activated by guanine exchange factors (GEFs), such as dedicator of cytokinesis (DOCK) family members like DOCK2 and DOCK8.7 Biallelic mutations in DOCK2 result in early-onset invasive bacterial and severe viral infections and T-cell lymphopenia.8DOCK8 mutations also result in severe bacterial, viral, and fungal infections, as well as eczema and severe environmental allergies.9 These descriptions highlight the predominant role of DOCK protein members in immune-related actinopathies.10 A role of DOCK11 in human immune disease has been long suspected but, to the best of our knowledge, never described so far. Here, we have identified 7 hemizygous mutations in DOCK11, a member of the DOCK-D subfamily, in 8 patients with early-onset autoimmunity, including autoimmune cytopenia and systemic lupus erythematosus (SLE).
Methods
This section briefly describes the methods; further details are provided in the supplemental Appendix, available on the Blood website. Before the study, all patients signed informed consents approved by the CERAPH-Centre (IRB: #00011928). The biological samples are part of INSERM UMR1163/Imagine collection declared to the French Ministère de la Recherche (CODECOH no DC-2020-3994).
Protein structure analysis
Three-dimensional (3D) structure prediction of the DOCK11 protein was performed by combining information from literature-described structures and AlphaFold2 predictions.11 The detailed method is described in the supplemental Appendix.
Genetic and functional analysis
Whole-exome and Sanger sequencing, mass cytometry, enzyme-linked immunosorbent assay, and G-protein–linked immunosorbent assay, confocal microscopy, migration assays, and DOCK11 knockdown methods are detailed in the supplemental Appendix.
Statistical analysis
Statistical analyses were performed using GraphPad Prism6 (GraphPad, La Jolla, CA). Data were analyzed by one-way analysis of variance followed by a post hoc test, or unpaired nonparametric Mann-Whitney tests or Kruskal-Wallis tests as indicated in the figure legends. Differences were considered significant when P < .05.
Results
Clinical and biological features of the cohort
In total, 8 male patients (aged, 7-29 years) from 7 families of various ethnic origins were studied. Clinical and biological data are summarized in Table 1, supplemental Table 1, and supplemental Figure 1. Detailed clinical cases are in the supplemental Appendix.
All patients presented with early-onset autoimmunity at a median age of 5 years (range, 1-14 years). Five patients (A, C, E, F, and G) had autoimmune cytopenia (Table 1). Twin patients (D1 and D2) had SLE. Patient B had a severe rheumatoid factor–positive polyarticular juvenile arthritis and recurrent hemolytic anemia with positive Coombs tests. In addition, patient F had an autoinflammatory syndrome with lymphoproliferation (supplemental Appendix).
Five patients (A, C, D1, D2, and F) presented with various cutaneous manifestations (detailed in Table1; Figure 1A; supplemental Figure 1), and 4 patients (A, B, D1, and F) with digestive manifestations (Table 1; supplemental Figure 2). None of the patients presented with recurrent or severe infections, allergies, nor bleeding disorders.
DOCK11 hemizygous mutations: structural consequences and protein expression. (A) Dermatological clinical findings of patients with DOCK11 deficiency (from left to right): lobular panniculitis on feet in patient A, bullous cutaneous SLE in patient D2, and noninfectious ecthyma gangrenosum on external lateral border of the right foot in patient F. (B) Schematic overview of DOCK11 protein with the positions of patients’ DOCK11 mutations. Molecular partners described to interact with DOCK11 are mentioned below the domain they bind to. (C) 3D structure of human DOCK11, as predicted by AlphaFold2 (ribbon representation, except for DRH2, which is shown as a blue surface). Confidence is high (pLDDT > 90) for the individual domain 3D structures, whereas the 3D structure of the N- and C-terminal sequences, linkers between domains and large loops (often disordered) remain elusive (low pLDDT values indicated by asterisks). Two such very large loops are depicted with broken lines, with the amino acid intervals. Positions of the domains relative to each other also remain uncertain, except form the C2-DHR1 interface (very low PEA [predicted error alignment] values). Mutated amino acids are depicted in magenta (patients A, B, C, D, and F). D414 is predicted exposed at the surface of the C2 domain and likely to play a significant role in the C2-DRH1 interface (supplemental Figure 6A-C). L1298 is predicted with confidence as being buried with the hydrophobic core of the ARM repeats, conserved in DOCK-C proteins (Figure S6D). The region including H1336 and R1366, more variable and predicted with a lower confidence, is located at the concave surface of the ARM repeats, where interactions take place in complexes of other DOCK proteins with partners. (D) 3D structure of DHR-2 domain of human DOCK11, as predicted by AlphaFold2. The DHR-2 (in light blue) is represented to interact with CDC42 (in gray), as deduced from the superimposition of DOCK11 with the 3D structure of DOCK9 in complex with CDC42. Mutated amino acids are depicted in magenta and correspond to mutations in patients E and G. L1706 is in the hydrophobic core of DHR2 lobe A, important for dimerization. R1885 is part of DHR2 lobe B, which consists of 2 sheets predicted in direct contact with the switch 1 domain of CDC42. (E) DOCK11 expression was evaluated in activated T cells of HDs and patients (A, B, C, D1, F, and G) by western blotting. The graph shows the relative expression of DOCK11 vs HDs (set to 1) ± standard error of the mean (SEM) after normalization against Ku-70 expression.
DOCK11 hemizygous mutations: structural consequences and protein expression. (A) Dermatological clinical findings of patients with DOCK11 deficiency (from left to right): lobular panniculitis on feet in patient A, bullous cutaneous SLE in patient D2, and noninfectious ecthyma gangrenosum on external lateral border of the right foot in patient F. (B) Schematic overview of DOCK11 protein with the positions of patients’ DOCK11 mutations. Molecular partners described to interact with DOCK11 are mentioned below the domain they bind to. (C) 3D structure of human DOCK11, as predicted by AlphaFold2 (ribbon representation, except for DRH2, which is shown as a blue surface). Confidence is high (pLDDT > 90) for the individual domain 3D structures, whereas the 3D structure of the N- and C-terminal sequences, linkers between domains and large loops (often disordered) remain elusive (low pLDDT values indicated by asterisks). Two such very large loops are depicted with broken lines, with the amino acid intervals. Positions of the domains relative to each other also remain uncertain, except form the C2-DHR1 interface (very low PEA [predicted error alignment] values). Mutated amino acids are depicted in magenta (patients A, B, C, D, and F). D414 is predicted exposed at the surface of the C2 domain and likely to play a significant role in the C2-DRH1 interface (supplemental Figure 6A-C). L1298 is predicted with confidence as being buried with the hydrophobic core of the ARM repeats, conserved in DOCK-C proteins (Figure S6D). The region including H1336 and R1366, more variable and predicted with a lower confidence, is located at the concave surface of the ARM repeats, where interactions take place in complexes of other DOCK proteins with partners. (D) 3D structure of DHR-2 domain of human DOCK11, as predicted by AlphaFold2. The DHR-2 (in light blue) is represented to interact with CDC42 (in gray), as deduced from the superimposition of DOCK11 with the 3D structure of DOCK9 in complex with CDC42. Mutated amino acids are depicted in magenta and correspond to mutations in patients E and G. L1706 is in the hydrophobic core of DHR2 lobe A, important for dimerization. R1885 is part of DHR2 lobe B, which consists of 2 sheets predicted in direct contact with the switch 1 domain of CDC42. (E) DOCK11 expression was evaluated in activated T cells of HDs and patients (A, B, C, D1, F, and G) by western blotting. The graph shows the relative expression of DOCK11 vs HDs (set to 1) ± standard error of the mean (SEM) after normalization against Ku-70 expression.
Among the maternal carriers, patient B’s mother had metastatic melanoma and has been treated with immunotherapy; patient C’s mother died of colorectal cancer at age 52.
Hemizygous deleterious mutations in DOCK11
Seven different hemizygous missense variants of DOCK11 were identified by whole-exome sequencing (Figure 1B) in 7 unrelated and nonconsanguineous families. The 6 mothers who were tested, all carried the gene variants (supplemental Figure 3A). We assessed the expression of the mutant and wild-type alleles in CD4+ T cells, CD8+ T cells, B cells, and monocytes in 2 mothers. The expression of both DOCK11 alleles was similar, indicating a global random X-inactivation in T and B cells as well as in monocytes (supplemental Figure 3B). These mutations were predicted to be deleterious in silico, and 5 are private variants (supplemental Figure 3A). The mutated amino acids are located in distinct domains of the DOCK11 protein (Figure 1B). Prediction of DOCK11 protein 3D structure allowed us to apprehend mutations’ potential deleterious impact (Figure 1C-D; supplemental Figure 4) and suggests that the variants may cause abnormal protein folding or directly affect DOCK11 interactions with its partners. Finally, DOCK11 expression was variably reduced in lysates from patients’ activated T cells (Figure 1E).
DOCK11 mutations impair platelet function and morphology
Platelets play a central role in immune thrombocytopenia (ITP) and participate in the immune dysregulation in SLE pathogenesis.12 Because 4 patients presented with ITP, and 2 patients with SLE, we investigated the impact of DOCK11 mutations on platelet function.
A significant decrease in the expression of DOCK11 was observed in platelets of patients compared with those of healthy donors (HDs) (Figure 2A).
Study of platelets from patients with mutated DOCK11. (A) DOCK11 expression was evaluated in platelets of HDs, and patients (A, C, D1, D2, F and G) by western blotting. Dotted lines indicate that the samples were derived from the same gel but were noncontiguous. The graph shows the mean of the relative expression of DOCK11 vs HD (set to 1) ± SEM after normalization against β-actin expression from several independent experiments (HDs, n = 69; A, n = 22; C, n = 14; G, n = 16; D1, D2, n = 8; F, n = 15). Statistical difference was evaluated by one-way analysis of variance (ANOVA) with Dunnett correction test for multiple comparisons (∗P < .05; ∗∗∗P < .001). (B) Platelet count for each patient (A, C, D1, D2, F, and G) was determined by automated blood cell counter. Shaded area represents the normal range between 150 × 109 and 400 × 109 platelets per L. Patients A and C were investigated for their platelet count several times (2 and 3 times, respectively). (C) Platelet size was evaluated by flow cytometry. Each dot represents the mean size measured by the mean forward scatter height (FSC-H; a.u., arbitrary units) of washed platelets from HDs and patients./ The box-and-whisker plot shows the normal range, determined from HDs. Whiskers represent the 5th to 95th percentiles, the box correspond to the interquartile range, the center line is the median, and the cross indicates the mean of 65 platelets. (D) Platelet ultrastructure, which was analyzed once for each patient using transmission electron microscopy (TEM). Scale bar represents 1 μm. (E-F) TEM analysis of platelet ultrastructure in each patient. Platelet surface (E) and platelet shape (F), which is defined as the ratio between the largest diameter and the smallest diameter, are derived from the TEM images. Graphs represent the mean ± SEM of at least 1100 HD platelets and 100 patient platelets. Statistical significance was determined in a one-way ANOVA with the Dunnett posttest for multiple comparisons. (G) Graph represents the mean percentage ± SEM of discoid platelets (dark gray), platelets with filopodia (light gray) and platelets with lamellipodia or full spreading (white) of at least 150 analyzed platelets from several independent experiments (HDs, n = 17; patient A, n = 4; patient C, n = 3). Statistical significance was determined only for patients A and C compared with HDs in a one-way ANOVA with the Dunnett posttest for multiple comparisons (∗P < .05; ∗∗P < .01). Patients D1, D2, F, and G were not statistically analyzed because only 1 experiment was performed. Scale bar represents 10 μm. (H) Spreading of HD and patient platelets onto fibrinogen matrix for 30 minutes was analyzed by epifluorescence microscopy in the presence of apyrase (5 U/mL) and indomethacin (4.5 μM). Platelet morphology was visualized using F-actin detection by fluorescently labeled phalloidin. (I) CDC42 activity evaluated in HD platelets and patient platelets by G-LISA after stimulation by 0.5 U/mL thrombin for 1 minute in unstirred conditions. Graph represents the relative CDC42 activity of each patient compared with that of HDs (set to 100%). G-LISA, G-protein–linked immunosorbent assay.
Study of platelets from patients with mutated DOCK11. (A) DOCK11 expression was evaluated in platelets of HDs, and patients (A, C, D1, D2, F and G) by western blotting. Dotted lines indicate that the samples were derived from the same gel but were noncontiguous. The graph shows the mean of the relative expression of DOCK11 vs HD (set to 1) ± SEM after normalization against β-actin expression from several independent experiments (HDs, n = 69; A, n = 22; C, n = 14; G, n = 16; D1, D2, n = 8; F, n = 15). Statistical difference was evaluated by one-way analysis of variance (ANOVA) with Dunnett correction test for multiple comparisons (∗P < .05; ∗∗∗P < .001). (B) Platelet count for each patient (A, C, D1, D2, F, and G) was determined by automated blood cell counter. Shaded area represents the normal range between 150 × 109 and 400 × 109 platelets per L. Patients A and C were investigated for their platelet count several times (2 and 3 times, respectively). (C) Platelet size was evaluated by flow cytometry. Each dot represents the mean size measured by the mean forward scatter height (FSC-H; a.u., arbitrary units) of washed platelets from HDs and patients./ The box-and-whisker plot shows the normal range, determined from HDs. Whiskers represent the 5th to 95th percentiles, the box correspond to the interquartile range, the center line is the median, and the cross indicates the mean of 65 platelets. (D) Platelet ultrastructure, which was analyzed once for each patient using transmission electron microscopy (TEM). Scale bar represents 1 μm. (E-F) TEM analysis of platelet ultrastructure in each patient. Platelet surface (E) and platelet shape (F), which is defined as the ratio between the largest diameter and the smallest diameter, are derived from the TEM images. Graphs represent the mean ± SEM of at least 1100 HD platelets and 100 patient platelets. Statistical significance was determined in a one-way ANOVA with the Dunnett posttest for multiple comparisons. (G) Graph represents the mean percentage ± SEM of discoid platelets (dark gray), platelets with filopodia (light gray) and platelets with lamellipodia or full spreading (white) of at least 150 analyzed platelets from several independent experiments (HDs, n = 17; patient A, n = 4; patient C, n = 3). Statistical significance was determined only for patients A and C compared with HDs in a one-way ANOVA with the Dunnett posttest for multiple comparisons (∗P < .05; ∗∗P < .01). Patients D1, D2, F, and G were not statistically analyzed because only 1 experiment was performed. Scale bar represents 10 μm. (H) Spreading of HD and patient platelets onto fibrinogen matrix for 30 minutes was analyzed by epifluorescence microscopy in the presence of apyrase (5 U/mL) and indomethacin (4.5 μM). Platelet morphology was visualized using F-actin detection by fluorescently labeled phalloidin. (I) CDC42 activity evaluated in HD platelets and patient platelets by G-LISA after stimulation by 0.5 U/mL thrombin for 1 minute in unstirred conditions. Graph represents the relative CDC42 activity of each patient compared with that of HDs (set to 100%). G-LISA, G-protein–linked immunosorbent assay.
Patient platelet counts were normal or subnormal, except for patients A and C, who presented mild to severe thrombocytopenia (Figure 2B) but without glycosylation profile defect (supplemental Figure 5A). Patient platelet size was also normal, except for patient A who exhibited macroplatelets (Figure 2C; supplemental Figure 5B). Moreover, electron microscopy ultrastructure analysis revealed abnormal morphology with elongated platelets in patients D1, D2, and G, which was in contrast with the discoid shape of resting platelets in HDs (Figure 2D-F).
Of 6 patients tested, 5 exhibited a defect of αIIbβ3 integrin activation at low concentrations of thrombin and convulxin (supplemental Figure 5C), indicating a thrombopathy. Of note, the expression of the main platelet receptors, glycoprotein Ibα, glycoprotein VI, and integrin αIIbβ3 was normal in patients, except for patient A whose integrin αIIbβ3 level was reduced (supplemental Figure 5D). Analysis of platelet morphologies during platelet adhesion on various matrixes (Figure 2G-H; supplemental Figure 5E) identified discoid platelets (resting stage), platelets with filopodia (intermediate morphological stage), and platelets with lamellipodia or full spreading (final morphological step).13 All patients exhibited an abnormal frequency of platelets at the final stage, except for patient D2, who exhibited more resting platelets. These results indicate that the patient platelets exhibit abnormal morphological modifications during platelet adhesion and spreading. Of note, platelet functional assays were also performed for 2 mothers. A normal integrin αIIbβ3 activation was observed after stimulation of platelets by thrombin or convulxin and normal platelet morphologies distributions after adhesion (supplemental Figure 6A-B). Because DOCK11 is a GEF activating CDC42,14 CDC42 activation was evaluated by G-protein–linked immunosorbent assay in patient platelets upon thrombin activation (Figure 2I). In all tested patients (A, D1, D2, F, and G), platelets exhibited impaired CDC42 activity, providing a rationale for the lower frequency of filopodia stage upon adhesion and suggesting that DOCK11 mutations are loss-of-function mutations. Predominant lamellipodia or full spreading of patient platelet suggested an impact on others Rho-GTPases, such as RhoA.15 In 2 patients tested (A and F), the RhoA activity was increased (supplemental Figure 5F). This was mimicked by pharmacological treatment with Cdc42 inhibitor of HD platelets. In this condition, the RhoA activity was also increased (supplemental Figure 5F). Taken together, these results indicated an imbalance in the CDC42/RHOA activity in patient cells.
DOCK11-mutated cells present abnormal morphological features
T-cell receptor-activated T cells from patients or HDs were spread on coverslips previously coated with poly-L-lysine, used as internal control, or with anti-CD28 and anti-CD3 antibodies at different concentrations (Figure 3A). On the antibodies-coated coverslips, activated T cells tended to increase their spreading area along with the concentration of anti-CD3 antibody. At a mild concentration (0.1 μg/mL), patient T cells showed reduced circularity and increased area, probably because of higher numbers of protrusions formed by DOCK11-mutated T cells (supplemental Figure 7A). At a higher anti-CD3 concentration (1 μg/mL), mutated DOCK11 T cells had a larger spreading area than HD T cells (Figure 3A). Notably, pharmacological activation of CDC42 restored cell spreading area to control levels (Figure 3A). These results were confirmed in activated T cells from HDs after DOCK11 short hairpin RNA (shRNA) inactivation (supplemental Figure 7B).
DOCK11 mutations lead to abnormal cell morphologies. Spreading assays with different lymphocytes subsets. (A) T-cell morphologies after spreading. Blue color corresponds to 4′,6-diamidino-2-phenylindole staining (nucleus). Red color corresponds to actin staining. Each patient is represented by a different symbol: patient A (⁕), patient B (●), patient C (■), patient D1 (×), patient D2 (★), patient F (∇), and patient G (o). Coverslips were coated with either poly-L-lysine (i); poly-L-lysine, anti-CD28, and anti-CD3 (0.1 μg/mL) antibodies (ii); or poly-L-lysine, anti-CD28, and anti-CD3 (1 μg/mL) antibodies (iii). Scale is set at 10 μm. Cell area was measured with or without CN02 treatment (CDC42/RAC1 activator). Nonparametric Kruskal-Wallis test was performed (∗P < .05). (B) B-lymphoblastic cell lines morphologies after spreading. Several morphologies were observed: cells with no protrusion, cells spread along 1 axis, cells with fine protrusions (filopodia), cells with lamellipodia, and cells presenting both lamellipodia and filopodia. Cells from patients presented abnormal protrusions. Repartition of each morphology types is shown. Scale is set at 10 μm. (C) Morphologies of mature MDDCs from HD, using either a scramble shRNA or a DOCK11-shRNA, after stimulation with lipopolysaccharide (LPS) 100 ng/mL for 24 hours, were observed using scanning electron microscopy. Scale is set at 5 μm.
DOCK11 mutations lead to abnormal cell morphologies. Spreading assays with different lymphocytes subsets. (A) T-cell morphologies after spreading. Blue color corresponds to 4′,6-diamidino-2-phenylindole staining (nucleus). Red color corresponds to actin staining. Each patient is represented by a different symbol: patient A (⁕), patient B (●), patient C (■), patient D1 (×), patient D2 (★), patient F (∇), and patient G (o). Coverslips were coated with either poly-L-lysine (i); poly-L-lysine, anti-CD28, and anti-CD3 (0.1 μg/mL) antibodies (ii); or poly-L-lysine, anti-CD28, and anti-CD3 (1 μg/mL) antibodies (iii). Scale is set at 10 μm. Cell area was measured with or without CN02 treatment (CDC42/RAC1 activator). Nonparametric Kruskal-Wallis test was performed (∗P < .05). (B) B-lymphoblastic cell lines morphologies after spreading. Several morphologies were observed: cells with no protrusion, cells spread along 1 axis, cells with fine protrusions (filopodia), cells with lamellipodia, and cells presenting both lamellipodia and filopodia. Cells from patients presented abnormal protrusions. Repartition of each morphology types is shown. Scale is set at 10 μm. (C) Morphologies of mature MDDCs from HD, using either a scramble shRNA or a DOCK11-shRNA, after stimulation with lipopolysaccharide (LPS) 100 ng/mL for 24 hours, were observed using scanning electron microscopy. Scale is set at 5 μm.
Subsequently, we analyzed morphologies of B-lymphoblastoid cell lines (B-LCLs) after spreading on anti-CD44–coated coverslips (Figure 3B). In these conditions, some B-LCL cells from patients exhibited an abnormal morphology, with numerous fine and long protrusions (Figure 3B).
Finally, DOCK11 shRNA inactivation was also performed in monocyte-derived dendritic cells (MDDCs) from HDs. DOCK11 expression was reduced by almost 80% at the messenger RNA and protein levels (supplemental Figure 7B). After activation and spreading, DOCK11-inactivated MDDCs had larger and more prominent protrusions (supplemental Figure 8A-B) and produced more interleukin-1β (IL-1β) cytokine (supplemental Figure 8C). Moreover, ruffles were aberrant and not comparable with the cells transfected with the scramble shRNA (Figure 3C).
Finally, primary skin-derived fibroblasts from patient A revealed abnormal protrusions compared with HD fibroblasts (supplemental Figure 9).
Overall, our results suggest that DOCK11 deficiency impairs the morphology of several cell lineages.
DOCK11 mutations affect leukocyte migration, and polarization of actin cytoskeleton under confinement
To confirm that DOCK11 variants impair actin cytoskeleton dynamics in lymphocytes, we compared the spontaneous migratory capacity of activated T and B-LCL cells from HDs and patients with DOCK11 (A-G) under confinement (Figure 4A).
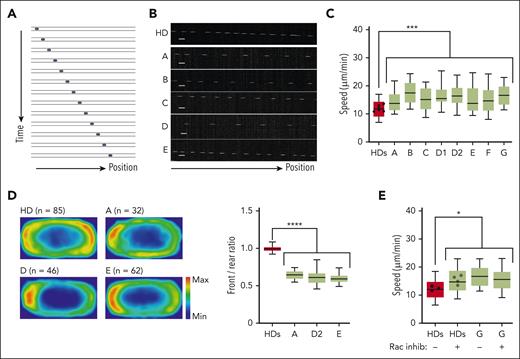
DOCK11 is needed for human leukocytes migration under confinement. Analysis of human HD and DOCK11-deficient T-cell migration in fibronectin-coated microchannels. Boxes include the 80% of the points, and bars represent the higher and lower 10% of points. (A) Representative montage of the change of position over time of human T-cell blast migrating inside microchannels of 4 × 5 μm with a timelapse of 2 minutes. (B) Representative kymograph of T-cell blast from HD (top panel) and DOCK11 patients (A-E, lower panels as indicated). (C) Mean instantaneous speed of HD and DOCK11 T-cell blasts migrating in 4 × 5 μm microchannels. Results from n = 2 independent experiments with each condition. Unpaired nonparametric Mann-Whitney test was used to evaluate statistical significance between the mean speeds of cells from controls and patients (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). (D) Density maps representing the enrichment of actin in HD and DOCK11-deficient T cells migrating in 8 × 5 μm microchannels. Cells were allowed to migrate, fixed with 4% paraformaldehyde (PFA) to maintain the polarized morphology, and stained with phalloidin to visualize F-actin. The density maps were generated by averaging the signal from HD (n = 85), patient A (n = 32), patient D (n = 46), and patient E (n = 63) cells. Quantification of the signal intensity on density maps presented as front/rear ratio per cell. One-way ANOVA was used to evaluate statistical significance. (E) Mean instantaneous speed of HD and DOCK11 T-cell blasts (patient G) migrating in 4 × 5 μm microchannels, with or without RAC inhibitor treatment. Results from n = 2 independent experiment with each condition. One-way ANOVA test was used to evaluate statistical significance. Unpaired nonparametric Mann-Whitney test was used to evaluate statistical significance between the mean speeds of cells from controls and treated controls, patients and patients who were treated (∗P < .05).
DOCK11 is needed for human leukocytes migration under confinement. Analysis of human HD and DOCK11-deficient T-cell migration in fibronectin-coated microchannels. Boxes include the 80% of the points, and bars represent the higher and lower 10% of points. (A) Representative montage of the change of position over time of human T-cell blast migrating inside microchannels of 4 × 5 μm with a timelapse of 2 minutes. (B) Representative kymograph of T-cell blast from HD (top panel) and DOCK11 patients (A-E, lower panels as indicated). (C) Mean instantaneous speed of HD and DOCK11 T-cell blasts migrating in 4 × 5 μm microchannels. Results from n = 2 independent experiments with each condition. Unpaired nonparametric Mann-Whitney test was used to evaluate statistical significance between the mean speeds of cells from controls and patients (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). (D) Density maps representing the enrichment of actin in HD and DOCK11-deficient T cells migrating in 8 × 5 μm microchannels. Cells were allowed to migrate, fixed with 4% paraformaldehyde (PFA) to maintain the polarized morphology, and stained with phalloidin to visualize F-actin. The density maps were generated by averaging the signal from HD (n = 85), patient A (n = 32), patient D (n = 46), and patient E (n = 63) cells. Quantification of the signal intensity on density maps presented as front/rear ratio per cell. One-way ANOVA was used to evaluate statistical significance. (E) Mean instantaneous speed of HD and DOCK11 T-cell blasts (patient G) migrating in 4 × 5 μm microchannels, with or without RAC inhibitor treatment. Results from n = 2 independent experiment with each condition. One-way ANOVA test was used to evaluate statistical significance. Unpaired nonparametric Mann-Whitney test was used to evaluate statistical significance between the mean speeds of cells from controls and treated controls, patients and patients who were treated (∗P < .05).
As previously described,16 human activated HD T cells presented constant trajectories with few directional changes (Figure 4B). In contrast, all T cells derived from patients were faster than their HD counterpart (Figure 4C). Of note, T cells of patient B’s mother had a migration speed similar to that of HDs (supplemental Figure 6C). Similarly, patient-derived B-LCLs had higher migration speeds (supplemental Figure 10A). To determine whether the increased speed of DOCK11-deficient cells correlated with alterations in actin polymerization, we assessed the front-rear distribution of actin in T cells fixed during migration in microchannels. In agreement with previous work,16 HD cells presented a bimodal actin distribution, at the front and the rear of the cells (Figure 4D). In contrast, in patients who were tested, DOCK11-deficient cells accumulated more actin at the rear of the cell (ie, reduced front/rear actin ratio), consistent with the increased speed of these cells (Figure 4C-D).
Mechanistically, the motility of activated T cells in microchannels depends on the balance in actin polymerization at the front (reflecting CDC42 activity) and rear (reflecting RHOA activity) of the cell.17 These results were also observed in activated T cells from HDs after DOCK11 shRNA inactivation (supplemental Figure 10C).
Considering that DOCK11 has been associated with CDC42 activation,7,14 we hypothesized that DOCK11 defects could reduce the activity of actin nucleators at the leading edge, which would displace the balance of actin polymerization and increase polymerization at the rear of the cells (thus increasing their speed). To test this hypothesis, HD T cells were treated with a pharmacological RAC1/CDC42 inhibitor. This treatment increased the speed of control T cells and impaired the front-rear ratio of actin polarization, recapitulating the alterations displayed by the DOCK11-deficient cells (Figure 4E; supplemental Figure 10D). Altogether our results suggest that DOCK11 is a critical regulator of the actin cytoskeleton dynamics and leukocytes migration under confinement.
DOCK11 deficiency results in abnormal Tregs phenotype and STAT5B activation in lymphocytes
Next, we analyzed the impact of DOCK11 deficiency on the immune phenotype and some immune functions. Flow cytometry immunophenotyping revealed variable alterations of circulating immunoglobulins and B-cell subsets, especially increased frequencies of CD21low and transitional B cells (Table 1; supplemental Table 1). Elevated concentrations of a proliferation inducing ligand (APRIL) and B-cell activating factor (BAFF) were found in plasma from patients (supplemental Figure 11). Elevated plasma concentrations of inflammatory cytokines were also observed in most patients (supplemental Figure 11). Mass cytometry immunophenotyping on whole blood did not reveal major alterations of cell subsets except for reduced proportions of early and late natural killer cells, mucosal-associated invariant T (MAIT) cells, and memory B cells (Figure 5). Of note, CD57 expression was significantly increased in CD4+ T cells and γδ T cells (supplemental Figure 12).
Immune phenotype by mass cytometry. (A) Uniform manifold approximation and projection (UMAP) of immune cell subset clusters analyzed by mass cytometry on whole blood samples from DOCK11 patients (n = 7) and age-matched HDs (n = 6). Each cluster is color coded. (B) Cell cluster biases observed between DOCK11 patients and HDs. DOCK11 patients have a significantly lower percentage of CD56bright natural killer (NK) cells, late NK cells, MAIT cells, and memory B cells. Two-tailed P values were determined with a nonparametric Mann-Whitney test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005.
Immune phenotype by mass cytometry. (A) Uniform manifold approximation and projection (UMAP) of immune cell subset clusters analyzed by mass cytometry on whole blood samples from DOCK11 patients (n = 7) and age-matched HDs (n = 6). Each cluster is color coded. (B) Cell cluster biases observed between DOCK11 patients and HDs. DOCK11 patients have a significantly lower percentage of CD56bright natural killer (NK) cells, late NK cells, MAIT cells, and memory B cells. Two-tailed P values were determined with a nonparametric Mann-Whitney test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005.
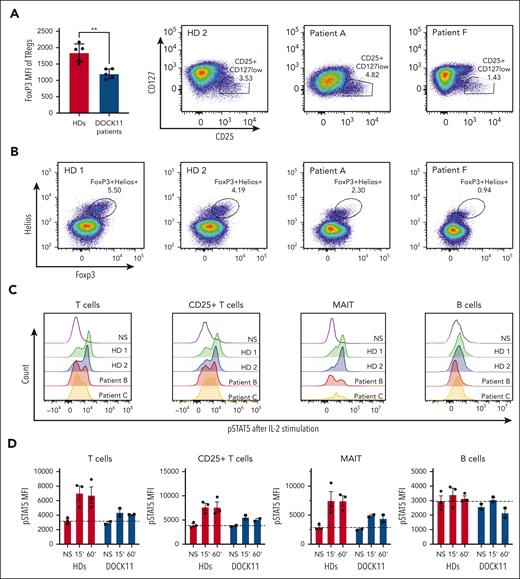
Autoimmune manifestations were prominent in all patients. In some cases, severe autoimmune enteropathy, reminiscent of the clinical presentation of FOXP3 deficient patients, suggested possible regulatory T-cell (Tregs) dysfunction.18 Tregs were first analyzed using the CD4+CD25+CD127low surface markers. Despite a slight decrease in patient F, the proportion of this subset in the 2 other patients tested is comparable with that in the HD controls (Figure 6A). Conversely, we observed a substantial reduction in the expression of FOXP3, and to a lesser extent, of HELIOS, by analyzing intracellular staining (Figure 6B). Therefore, the proportion of activated Tregs defined as CD4+ FOXP3+ HELIOS+, was lower in these patients compared with in controls (Figure 6B). These results suggested that DOCK11 could be involved in the regulation of FOXP3 in Tregs. Among multiple factors regulating the FOXP3 transcription, STAT5B activation has been described as a main regulator of FOXP3.19 Consistent with the reduced FOXP3 expression in Tregs, we found a reduced activation of STAT5B in patient T cells compared with HDs upon IL-2 stimulation (Figure 5C-D). In contrast, we observed a normal, or slightly reduced, IL-6–dependent STAT3 activation in the patient cells, suggesting a major impact of DOCK11 deficiency on the IL-2–STAT5 pathway only (supplemental Figure 13C). A decreased IL-2–induced STAT5B activation was also observed in B lymphocytes and MAIT cells (Figure 5C-D). More importantly, we confirmed a decreased IL-2–induced STAT5B activation in T cells from patients (supplemental Figure 13A) and from HDs, in which we induced a decrease in DOCK11 expression by knockdown (supplemental Figure 13B). Consistently, we also observed a variably reduced proliferation upon CD3 stimulation in activated T cells from 5 patients compared with HDs (supplemental Figure 14). Overall, these results suggest that the DOCK11 deficiency is associated with impairment of the IL-2–STATB pathway in different patients’ lymphocyte subsets, as well as in DOCK11-knockdown T cells from HDs.
Treg phenotype and STAT5 activation. (A) FoxP3 mean fluorescence intensity on Tregs from HDs and patients with DOCK11. Treg are defined by CD4+ CD25+ CD127low as depicted in the graph on the right side of the panel. (B) Representative dot plots of CD3+ CD4+ Foxp3+ Helios+ cells from HDs and 2 patients with DOCK11. (C) Representative histograms of phospho-STAT5 in T cells, CD25+ T cells, and B cells from HDs and patients with DOCK11. Cells were stimulated for 60 minutes with IL-2. Results for unstimulated (NS) cells are shown with the black peak. (D) Kinetics of phospho-STAT5 expression upon IL-2 stimulation: unstimulated (NS), 15 and 60 minutes. Mean fluorescence intensity (MFI) of phospho-STAT5 in T cells, CD25+ T cells, MAIT cells, and B cells from HDs and patients with DOCK11.
Treg phenotype and STAT5 activation. (A) FoxP3 mean fluorescence intensity on Tregs from HDs and patients with DOCK11. Treg are defined by CD4+ CD25+ CD127low as depicted in the graph on the right side of the panel. (B) Representative dot plots of CD3+ CD4+ Foxp3+ Helios+ cells from HDs and 2 patients with DOCK11. (C) Representative histograms of phospho-STAT5 in T cells, CD25+ T cells, and B cells from HDs and patients with DOCK11. Cells were stimulated for 60 minutes with IL-2. Results for unstimulated (NS) cells are shown with the black peak. (D) Kinetics of phospho-STAT5 expression upon IL-2 stimulation: unstimulated (NS), 15 and 60 minutes. Mean fluorescence intensity (MFI) of phospho-STAT5 in T cells, CD25+ T cells, MAIT cells, and B cells from HDs and patients with DOCK11.
Discussion
We identified 7 private and rare hemizygous missense mutations of DOCK11 leading to early-onset autoimmune disease. To the best of our knowledge, human DOCK11 deficiency has never been described thus far. DOCK11 is a GEF protein of the DOCK-D family. Mostly expressed in hematopoietic cells, DOCK11 is suspected to affect actin cytoskeleton remodeling through CDC42 activation, an important Rho-GTPase.8,16
All DOCK11 mutations are predicted to be deleterious, and 3D modeling suggests that these mutations could affect the stability of the protein conformation or its interactions with putative partners. Accordingly, reduced DOCK11 expression was observed in patients' platelets and lymphocytes. The different variants, leading to a variable protein expression, may account for the variable clinical phenotypes observed in patients. Nevertheless, all patients who were tested showed reduced CDC42 activity in platelets, highlighting the abnormal function of the DOCK11 mutants. Decreased CDC42 activity in platelets was associated with impaired platelet morphology, spreading, and activation. The spreading of platelets implicates Rho-GTPases such as CDC42, RAC, and RHO via integrin activation.13,15 Some studies suggested that CDC42 is involved in platelet filopodia formation and spreading on fibrinogen.20 RhoA activity, implicated in lamellipodia and stress fiber formation of platelets, was increased in the patients tested and increased after inhibition of CDC42 in platelets of HDs. Stimulated platelets from patients had a higher proportion of spread platelets, with deficient filopodia formation, reflecting a potentially disrupted balance between the Rho-GTPases CDC42 and RhoA.
Of note, reduced integrin αIIbβ3 activation was observed in patient platelets upon stimulation with an agonist at low concentration but not at high concentration. These results are consistent with a partial impairment of CDC42 activation in patient platelets.21 Interestingly, circulating anti-αIIbβ3 antibodies were detected in patient A, who developed severe ITP with highly fluctuating platelet counts. The absence of significant bleeding disorder in DOCK11-deficient patients, with or without ITP, suggests that the hemostatic role of platelets is not affected. Platelets extensive exploration highlighted impaired actin cytoskeleton, abnormal activation, and disrupted Rho-GTPase balance in all patients tested, regardless of the presence or absence of thrombocytopenia. Functional nonhemostatic thrombopathy may impair the immunoregulation role of platelets as described in other autoimmune diseases.12,22,23
T- and B-cell development and function can be disrupted in immune-related actinopathy, as previously described in other DOCK deficiencies.24-26 Patients' T cells and DOCK11-knockdown activated T cells from healthy controls exhibited aberrant cytoskeleton rearrangement with larger spreading areas and more protrusions, which could impair migration, sensing, or interactions with other immune cells.27,28 Indeed, migration speed under confinement was increased concomitantly with an abnormal actin polymerization at the rear during migration of the patient activated T cells and in the DOCK11-knockdown model, a finding consistent with an impaired balance in the activity of Rho-GTPases.29 Moreover, the pharmacological inhibition of RAC/CDC42 in HD-derived T cells reproduced the aberrant spreading, faster migration, and impaired actin polarization, as observed in T cells derived from patients. Conversely, incubating the patients' T cells with a CDC42 activator restored normal spreading. Taken together, these results support the conclusion that DOCK11 deficiency impairs the balance between CDC42 and RHOA activity in activated T cells, as observed in platelets. Interestingly, a faster migration was also observed in patients' B-LCL, indicating that DOCK11 would be a general regulator of lymphocyte migration. Lastly, we observed aberrant protrusions and ruffles upon reduced protein expression by DOCK11 knockdown in MDDCs from HDs. This is reminiscent of the role of mouse DOCK11 (Zizimin2) in the actin remodeling of bone marrow–derived dendritic cells.30 The abnormal cell shape of DOCK11-inactivated MDDCs, and their increased production of IL-1β suggested that the patients' dendritic cells could contribute to the immune dysregulation observed in these individuals.
The patients’ immunological phenotyping showed an abnormal distribution of the B-cell subsets with increased circulating BAFF and APRIL in the patients' plasma. Impaired balance of B-cell populations in favor of transitional and CD21low B cells with elevated cytokine levels of the BAFF complex may promote autoimmunity in patients, as already described in other immune disorders.31 The DOCK11-deficient mouse model (known as the zizimin-2 knockout, the mouse counterpart of DOCK11) showed disturbed B-cell subset distribution.32 Surprisingly, these mice did not develop autoimmune manifestations. However, many examples of genetic defects lead to very different phenotypes in humans and mice. For example, Lrba knockout in mice has no impact on regulating the immune response, whereas patients who are LRBA deficient present with primary immune deficiency and a broad spectrum of autoimmune manifestations.33,34
T-cell subset distribution was not overtly disturbed except for a reduced proportion of MAIT cells. Natural killer-cell subsets were also low in patients but without obvious clinical consequences such as viral infections or macrophage activation syndrome. Immunosenescence of T cells was increased in patients, consistent with the immune-aging role of DOCK11 suggested by the mouse models.30,32 Increased proinflammatory Th1 and Th17 cytokines in patients' plasma, without any documented infection, suggested a possible immune dysregulation that could be the cause, or the consequence, of the patients’ autoimmune and autoinflammatory phenotypes.
All patients presented with various clinical phenotypes, including severe autoimmune cytopenia, SLE, autoinflammatory syndrome, or lymphoproliferation. Most patients had inflammatory bowel disease, and some had various immune-related skin manifestations. Importantly, none of the patients had severe or recurrent infections, and vaccinal responses were normal in the 2 patients who were tested, indicating an overall normal humoral response.
The severe early-onset autoimmune enteropathy observed in some patients was reminiscent of the prominent symptoms observed in patients who are FOXP3 deficient, suggesting a potential Treg defect in the patients with DOCK11 deficiency.18 Although we observed a mildly reduced proportion of Tregs in 1 patient, we found the expected proportion of this subset in the remaining patients. However, more strikingly, we found a markedly decreased expression of FOXP3, the master Treg transcription factor, and HELIOS, to a lesser extent. These 2 transcription factors are essential to sustain the Treg suppressive functions.35,36 Moreover, Tregs are highly dependent of IL-2 and its downstream signaling pathway, as observed in IL-2, IL-2RA, IL2RB, and STAT5B deficiencies,37,38 all characterized by impaired Treg function and overt autoimmune manifestations. Interestingly, we observed a reduced STAT5B activation in the patient T and B cells upon IL-2 stimulation. This was corroborated by a slight, but consistent, reduction in T-cell proliferation. More importantly, the reduced IL-2–induced STAT5B activation is observed in DOCK11-knockdown T cells from healthy controls. All these results point to a global IL-2–STAT5 signaling impairment in the context of DOCK11 deficiency and provide a molecular basis for the underlying mechanisms leading to autoimmunity in these patients.
A challenging observation is that patients with DOCK11 develop overt autoimmune manifestations but not viral, bacterial, or fungal infections, whereas patients with DOCK8 patients predominantly present with recurrent sinopulmonary and mucocutaneous infections, severe allergy with elevated serum levels of immunoglobulin E but mild or any autoimmune symptoms.39 Nevertheless, both defects are leading to a reduced CDC42 activity,40 which therefore seems to be the main cause of the loss of cell shape integrity and abnormal motility, and indicates that both GEFs are nonredundant and possibly working in concert in this process. This also indicates that the impaired CDC42 activation is not fully involved in the pathophysiological mechanisms of each defect. The list of DOCK partners is probably far from being wholly defined. Because DOCK8 and DOCK11 belong to different DOCK subfamilies, one can anticipate that they may share common but also different partners. In addition, their respective expression may differ based on immune cell subsets. In humans, DOCK8 deficiency is reportedly associated with a reduced STAT3 activation in various lymphocyte subsets, consistent with the observation that patients who are DOCK8 deficient share common clinical and biological features with those who are STAT3 deficient, such as hyper immunoglobulin E, food allergy, and mucocutaneous infections related to impaired Th17 function.41 In this study we uncovered an IL-2–STAT5 (but not an IL-6–STAT3) signalization defect and an abnormal Treg phenotype, 2 findings that are consistent with the development of autoimmunity. Surprisingly, a similar Treg dysfunction has also been described in patients who are DOCK8 deficient, despite the lack of prominent autoimmunity, further blurring the understanding of the mechanisms involved.42 Very interestingly, although mice that are DOCK8 deficient have impaired Treg function but do not develop autoimmunity, mice with Treg-specific DOCK8 deficiency have lymphoproliferation, autoantibodies production, and enteropathy,43 thus suggesting that DOCK8 deficiency in conventional T cells, or maybe in other immune subsets, protects against the onset of autoimmunity in patients.
In contrast, one might speculate that the impairment of some immune subsets, such as the MAIT cells, for instance, or that the platelet abnormalities observed in patients with DOCK11 deficiency could contribute to the development of autoimmunity. Lastly, the DOCK11 expression pattern is not restricted to the hematopoietic compartment but also allows its expression in various skin and intestinal cells.44 Accordingly, we observed aberrant protrusions of primary skin fibroblasts derived from patient A, suggesting an impact on the actin cytoskeleton remodeling in nonhematopoietic cells. Therefore, it cannot be excluded that DOCK11 deficiency in nonhematopoietic cells contributes to tissue inflammation, possibly driving the autoimmune response to these tissues.
Another sharp contrast with the DOCK8 deficiency is the lack of overt viral infection despite the impact of DOCK11 deficiency on T-cell migration and possibly other immune cells, suggesting that DOCK11-deficient T cells could convey a normal tissue surveillance, possibly in line with a normal, or only slightly reduced, STAT3 activation. In addition to differential expression in immune cells, this might also relate to DOCK11-specific functions. Recently, Ide et al identified DOCK11 as a potential therapeutic target to prevent persistent hepatitis B virus infection45; the authors suggested that DOCK11 plays a role in the retrograde trafficking in the hepatitis B virus infection and contributes to maintaining covalently closed circular DNA. If this mechanism holds for other Herpes viruses, DOCK11 deficiency might passively protect against such viral infections.
In conclusion, this study describes a new X-linked immune-related actinopathy in 8 patients with severe early-onset autoimmunity, including SLE, caused by DOCK11 hemizygous mutations. Platelets, and T and B cells from patients who are DOCK11 deficient showed aberrant cytoskeleton remodeling with impaired CDC42 activity. The regulation of FOXP3 in Tregs is impaired, involving the IL-2–STAT5B pathway. Our results underscore the crucial DOCK11 function in hematopoietic cells, and provide a novel example of the implication of actin cytoskeleton in human immune disorders. Further analyses are warranted to better understand the complex interactions between DOCK11 and other DOCK molecules, notably DOCK8, as well as to better characterize the molecular link with the IL-2–STAT5B pathway. This can provide key information to help clinicians to adopt the best therapeutic options. All patients have been treated with different immunosuppressive drugs that variably controlled the autoimmune manifestations. Considering that DOCK11 deficiency affects cell shape, the mechanical migration, or the adhesion of circulating cells, T regulators, and possible tissue-resident immune cells, allogenic hematopoietic stem cell transplantation could be contemplated as a therapeutic option.
Limits of the study
All patients presented with autoimmune features that may reflect biased recruitment in our laboratory. Furthermore, the identified mutations seem to confer a partial DOCK11 defect. Therefore, we do not exclude that DOCK11 mutations could be associated with a broader or more severe clinical phenotype, including autoinflammatory diseases,40-42 or combined immune deficiencies, notably in patients with a complete DOCK11 deficiency.
Acknowledgments
The authors thank the patients and their families for participating in the study. The authors thank Noémie Paillon and Juan-Jose Saez-Pons from the laboratory of Claire Hivroz at Institute Curie, Université PSL, U932 INSERM, Integrative Analysis of T cell Activation Team, Paris, France, for the help with T-cell spreading assay. The authors thank the CIQLE Centre d’Imagerie Quantitative Lyon-Est (France) for expert technical assistance with the electron microscopy studies, and Dominique Lasne and Delphine Borgel at Necker Children’s Hospital (Hematology Department, Paris) for their assistance with platelet studies. In addition, the authors thank the staff from the Imagine genomic, bioinformatics and cytometry core facilities and the Salpétrière CyPS cytometry facility for advice and technical assistance. Lastly, the authors thank the attending physicians for their support and for coordinating the patients’ clinical care and sample collection. The graphical abstract was created with BioRender.com.
The study was funded by the INSERM, the French government (managed by the French National Research Agency) (Agence Nationale de la Recherche) through the “Investissements d’avenir” program (Institut Hospitalo-Universitaire Imagine, grant reference: ANR-10-IAHU-01; Recherche Hospitalo-Universitaire, grant reference: ANR-18-RHUS-0010), and other grants from the Agence National de la Recherche (ANR-18-CE17-0001 “Action,” ANR-18-CE15-0017), the Fondation pour la Recherche Médicale (grant reference: Equipe FRM EQU202103012670). The authors acknowledge the Centre de Référence Déficits Immunitaires Héréditaires, and the Centre de Référence des Cytopénies Auto-Immunes de l’Enfant (CEREVANCE), which allowed patient enrollment through the OBS’CEREVANCE and ACTION cohorts. This work was also supported by several grants. C. Boussard was a recipient of an INSERM “poste d’accueil” program and was awarded the prize of the Société Française de Pédiatrie. L.D. received a grant from the Imagine Thesis Award program. Q.R. received an Institut Imagine MD-PhD fellowship (funded by the Fondation Bettencourt Schueller) and a Société Nationale Française de Médecine Interne fellowship.
Authorship
Contribution: C. Boussard, L.D., T.G., A.F., A.K., F.A., F.E.S., I.C, and F.R.-L. conceptualized the study; I.C. and P.V. developed the methodology; C. Boussard, L.D., T.G., A.K., M.B., C. Brunaud, B.D, M.M.-N., J.S., C.R., J.-C.B., P.P., M.-C.S., O.P., A.M., V.P., P.V., S.D., A.V., M.Z., C.M., and M.R. performed investigations; L.D, N.G., I.C., Q.R. and C.M. were responsible for data curation; C. Boussard, L.D., I.C., T.G., P.P., and Q.R. performed formal analysis; C. Boussard, L.D., T.G, F.E.S., I.C., A.K., F.A., and F.R.-L. wrote the original manuscript draft; I.C., Q.R., N.A., P.D.A., M. Castelle, P.P., N.C.-B., F.C.-H., S.F., P.D., L.L., D.M., P.R., A.W.-M., M.R., B.N., J.-C.B., A.M., A.K., F.A., M.B., M.-C.S., C. Boussard, L.D., and F.R.-L. wrote, reviewed, and edited the manuscript; I.C., L.D., C. Boussard, Q.R., N.G., T.G., P.P., F.A., A.K., M.M.-N, B.D., J.-C.B., and T.G. were responsible for data visualization; C.L., N.A., P.D.A., B.B.-M., S.B.J., A.B., J.B., E.B., D.B., C. Bodemer, M. Chbihi, M.J., N.C.-B., F.C.-H., A.D., S.F., P.D., M.H., J.L.-P., L.L., P.R., F.R., M.T., A.W.-M., M.R., C.P., and B.N. provided resources; M.M., F.E.S., A.K., F.A., and F.R.-L. supervised the study; F.E.S. and F.R.-L. acquired funding for the study; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: L.D. is a former employee of Sanofi, France, and may hold shares and/or stock options in the company. The remaining authors declare no competing financial interests.
Correspondence: Frédéric Rieux-Laucat, Institut Imagine, 24 Blvd du Montparnasse, 75015 Paris, France; e-mail: frederic.rieux-laucat@inserm.fr.
References
Author notes
∗C. Boussard and L.D. contributed equally to this study.
†T.G., A.K., and M.B. contributed equally to this study.
‡M.M., F.E.S., F.A., and F.R.-L. are joint senior authors.
Data are available on request from the corresponding author, Frédéric Rieux-Laucat (frederic.rieux-laucat@inserm.fr).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![DOCK11 hemizygous mutations: structural consequences and protein expression. (A) Dermatological clinical findings of patients with DOCK11 deficiency (from left to right): lobular panniculitis on feet in patient A, bullous cutaneous SLE in patient D2, and noninfectious ecthyma gangrenosum on external lateral border of the right foot in patient F. (B) Schematic overview of DOCK11 protein with the positions of patients’ DOCK11 mutations. Molecular partners described to interact with DOCK11 are mentioned below the domain they bind to. (C) 3D structure of human DOCK11, as predicted by AlphaFold2 (ribbon representation, except for DRH2, which is shown as a blue surface). Confidence is high (pLDDT > 90) for the individual domain 3D structures, whereas the 3D structure of the N- and C-terminal sequences, linkers between domains and large loops (often disordered) remain elusive (low pLDDT values indicated by asterisks). Two such very large loops are depicted with broken lines, with the amino acid intervals. Positions of the domains relative to each other also remain uncertain, except form the C2-DHR1 interface (very low PEA [predicted error alignment] values). Mutated amino acids are depicted in magenta (patients A, B, C, D, and F). D414 is predicted exposed at the surface of the C2 domain and likely to play a significant role in the C2-DRH1 interface (supplemental Figure 6A-C). L1298 is predicted with confidence as being buried with the hydrophobic core of the ARM repeats, conserved in DOCK-C proteins (Figure S6D). The region including H1336 and R1366, more variable and predicted with a lower confidence, is located at the concave surface of the ARM repeats, where interactions take place in complexes of other DOCK proteins with partners. (D) 3D structure of DHR-2 domain of human DOCK11, as predicted by AlphaFold2. The DHR-2 (in light blue) is represented to interact with CDC42 (in gray), as deduced from the superimposition of DOCK11 with the 3D structure of DOCK9 in complex with CDC42. Mutated amino acids are depicted in magenta and correspond to mutations in patients E and G. L1706 is in the hydrophobic core of DHR2 lobe A, important for dimerization. R1885 is part of DHR2 lobe B, which consists of 2 sheets predicted in direct contact with the switch 1 domain of CDC42. (E) DOCK11 expression was evaluated in activated T cells of HDs and patients (A, B, C, D1, F, and G) by western blotting. The graph shows the relative expression of DOCK11 vs HDs (set to 1) ± standard error of the mean (SEM) after normalization against Ku-70 expression.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/22/10.1182_blood.2022018486/2/m_blood_bld-2022-018486-gr1.jpeg?Expires=1765926464&Signature=sfDQgQtLij4aoOcficL2QXVNGeU~zT4PD-C9p3ygOc-X~OYEthAky9gniMSuM~7DweQCu5bensMmmEK6VJOOPedjynUtQHw5MQdqTj2OOlHENYzU9fqrStGRdegulcZ7Z5rkXLLrwptP8OMY2Sn-Sg-vH2kzGtmcUMgiCRxKsuTOJFLqACxzjShYeA-QGtIntWIGiVeNtKrpCcylEwNp408p0XWF1Hz7pY7UMDVeOd2ohTKlbyk-GveyM6yEvfHm0y7NDjzoacnDDkY0LNDPNiZtKKMPdfiEliX927smUyM1T8~py5tV9IJ6EbBq33jb9wH7xuOvIoBqvZW4lcELKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![DOCK11 hemizygous mutations: structural consequences and protein expression. (A) Dermatological clinical findings of patients with DOCK11 deficiency (from left to right): lobular panniculitis on feet in patient A, bullous cutaneous SLE in patient D2, and noninfectious ecthyma gangrenosum on external lateral border of the right foot in patient F. (B) Schematic overview of DOCK11 protein with the positions of patients’ DOCK11 mutations. Molecular partners described to interact with DOCK11 are mentioned below the domain they bind to. (C) 3D structure of human DOCK11, as predicted by AlphaFold2 (ribbon representation, except for DRH2, which is shown as a blue surface). Confidence is high (pLDDT > 90) for the individual domain 3D structures, whereas the 3D structure of the N- and C-terminal sequences, linkers between domains and large loops (often disordered) remain elusive (low pLDDT values indicated by asterisks). Two such very large loops are depicted with broken lines, with the amino acid intervals. Positions of the domains relative to each other also remain uncertain, except form the C2-DHR1 interface (very low PEA [predicted error alignment] values). Mutated amino acids are depicted in magenta (patients A, B, C, D, and F). D414 is predicted exposed at the surface of the C2 domain and likely to play a significant role in the C2-DRH1 interface (supplemental Figure 6A-C). L1298 is predicted with confidence as being buried with the hydrophobic core of the ARM repeats, conserved in DOCK-C proteins (Figure S6D). The region including H1336 and R1366, more variable and predicted with a lower confidence, is located at the concave surface of the ARM repeats, where interactions take place in complexes of other DOCK proteins with partners. (D) 3D structure of DHR-2 domain of human DOCK11, as predicted by AlphaFold2. The DHR-2 (in light blue) is represented to interact with CDC42 (in gray), as deduced from the superimposition of DOCK11 with the 3D structure of DOCK9 in complex with CDC42. Mutated amino acids are depicted in magenta and correspond to mutations in patients E and G. L1706 is in the hydrophobic core of DHR2 lobe A, important for dimerization. R1885 is part of DHR2 lobe B, which consists of 2 sheets predicted in direct contact with the switch 1 domain of CDC42. (E) DOCK11 expression was evaluated in activated T cells of HDs and patients (A, B, C, D1, F, and G) by western blotting. The graph shows the relative expression of DOCK11 vs HDs (set to 1) ± standard error of the mean (SEM) after normalization against Ku-70 expression.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/22/10.1182_blood.2022018486/2/m_blood_bld-2022-018486-gr1.jpeg?Expires=1765926465&Signature=KIhucGlObEm8fZSySQbaRqmFULXsQW~FFJtxQFCKTi3s6KthBtA6KIQxsqzgB3WVvOE~PWY0FphBxSBbDIJ12ZZ6HmFG7Jkkq9kfJydl8OXwuf1lc2s7LXRX-6p9ipyogrZRSaFpNRK9Lp2jAX3qyjl5YcOATyWd5f7G0LNBoegwLcpHQQLkQyrXWWFnj-29KrIUF-BcTJz1xcVYrPhV42hEP1X~O668Y7baZ5Mm7Pknx8H-4Uc504f99XLO97LRVs6FflG~FCCnGsr9NQ50gorG7QR81MvInCbwYQtIV3nAp875totiM3YPAcDp8DwGS--RoKgfWHGvgvtsc27DSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)