In this issue of Blood, Daver et al1 report that idasanutlin and venetoclax therapy in refractory/relapsed AML results in responses in one-third of patients and in vivo expansion of preexisting p53 mutant clones.
Relapsed and refractory acute myeloid leukemia (R/R AML) carries a grim prognosis with survival measured in months.2 Outcomes are particularly dismal in patients whose disease lacks targetable mutations in FLT3, IDH1, or IDH2.3 The development of efficacious mutation-agnostic therapies to address this patient population remains a high priority, particularly in individuals with advanced age and multiple comorbidities who are ineligible for intensive regimens or allogeneic transplant.
In this article, Daver and colleagues describe the results of a phase 1b trial evaluating the safety, tolerability, and antileukemic activity of venetoclax combined with idasanutlin in older patients with R/R AML. This trial was conducted before the approval of venetoclax-based therapy for de novo AML in older patients and was based on compelling preclinical data and biological rationale.
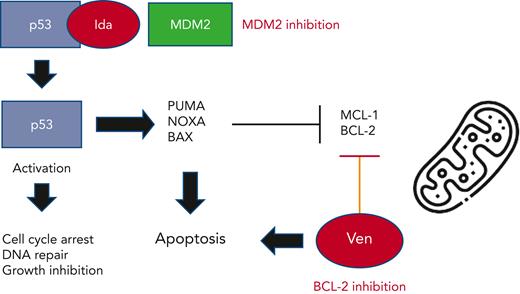
MDM2 (murine double minute 2) is a known negative regulator of the tumor suppressor gene, p53. Binding of MDM2 to p53 leads to its inactivation and degradation, thereby promoting uncontrolled cell proliferation and tumorigenesis. Idasanutlin is a potent and selective small molecule MDM2 antagonist which prevents MDM2 binding to p53.4 This leads to p53 protein stabilization and activation, resulting in cell cycle arrest, impaired DNA repair, and growth inhibition. In addition, p53 upregulates multiple proapoptotic factors (NOXA, BAX, PUMA) and downregulates antiapoptotic proteins including MCL-1 and BCL-2. Given these known mechanisms of action, the dual inhibition of MDM2 and BCL-2 could further enhance leukemia apoptosis (see figure). Multiple preclinical studies have confirmed the potent synergistic activity of combination BCL-2/MDM2 therapy in AML cells and xenograft models. Of note, these models typically employed p53 wildtype AML cells as functional p53 is considered critical for optimal MDM2 antitumor efficacy.5,6
Proposed mechanism of action of combined MDM2 (idasanutlin) and BCL-2 (venetoclax) inhibition on AML cells.
Proposed mechanism of action of combined MDM2 (idasanutlin) and BCL-2 (venetoclax) inhibition on AML cells.
Here in this phase 1b cohort, 55 older adults with R/R AML (including 10 patients with p53 mutant disease) were treated with different doses and durations of venetoclax and idasanutlin. Overall, the clinical efficacy was somewhat disappointing and does not appear to reflect the highly synergistic activity seen in preclinical studies. In previous trials, venetoclax monotherapy resulted in a CR/CRi rate of 19%,7 and idasanutlin monotherapy 18.9%.8 Here, the combination yielded a CRc rate of 26% (dose escalation) to 32% (dose expansion). Patients with IDH1, IDH2, or RUNX1 mutant R/R AML experienced higher response rates (45%-50%), perhaps reflecting enhanced sensitivity to BCL-2 inhibition plus MDM2 blockade. Although gastrointestinal toxicity, a potential treatment-limiting class effect of all MDM2 inhibitors, was common, implementation of standardized management strategies successfully limited severity. Nevertheless, the brevity of responses (median 3.9 months) and survival (median 5.1 months) appeared to belie any long-lasting clinical benefit.
Perhaps the most significant findings of this phase 1b trial involve the dynamics of p53 mutant AML on therapy. The presence of p53 did not preclude clinical benefit to MDM2 inhibition. Two patients with concomitant p53 and IDH1 or RUNX1 mutations achieved responses, supporting a hierarchy of molecular aberrations regulating responses. However, following therapy, 12 patients developed ≥1 emergent p53 mutations, the majority (88%) of which were detectable by deep sequencing at baseline. These emergent mutations were preferentially identified in responding patients, presumably due to the longer duration of selective therapeutic pressure. Additional studies to elucidate the frequency and kinetics of p53 mutation acquisition or expansion on MDM2 inhibitor therapy, are clearly warranted. It remains to be determined whether these mutant clones are innocent bystanders and/or constitute drivers of overt disease relapse. Of note, most patients with low-level p53 clones (<1% variant allele frequency [VAF]) at baseline did not have outgrowth, consistent with clonal hematopoiesis. In contrast, all patients with higher-level p53 disease burden (>1% VAF) experienced clonal expansion in study. Based on these findings, the use of ultra-deep sequencing to screen for preexisting p53 mutations in potential patients enrolling in MDM2 inhibitor trials remains an intriguing possibility.
Currently up to one-third of patients treated with BCL2/MDM2 inhibition in this study had evidence of emergent p53 and/or RAS mutant clones. It is uncertain whether halting idasanutlin and/or venetoclax would have decreased mutant VAF or led to the resolution of these aberrant clones. It is reasonable to assume that the dual antagonism of BCL-2 and MDM2 may have preferentially expanded the pool of p53 mutant clones given the known resistance of these cells to venetoclax therapy.9 Whether adding a third agent (such as azacitidine) to BCL-2 and MDM2 inhibition would mitigate this issue is unknown.
While idasanutlin is no longer being clinically developed for AML,10 alternative MDM2 inhibitors (eg, KRT-232, APG-115, siremadlin) remain under active investigation for myeloid malignancies. Data from this phase 1b trial of venetoclax and idasanutlin provide important insights into the potential benefits and pitfalls of MDM2 inhibition in AML patients to further optimize these and future clinical studies.
Conflict-of-interest disclosure: E.S.W. has served in an advisory capacity for AbbVie/Genentech and on data safety monitoring committees for AbbVie clinical trials.