Abstract
Introduction: Sickle cell anemia (SCA) is most prevalent in sub-Saharan Africa where few resources exist to address acute or chronic complications. SCA-related pediatric strokes are especially devastating, and lack of a reliable, affordable, and safe blood supply prohibit implementation of chronic transfusions. Transcranial Doppler (TCD) ultrasound for primary stroke risk assessment coupled with hydroxyurea, escalated to maximum tolerated dose (MTD), to decrease stroke risk could be transformative for children with SCA in Africa. Hydroxyurea is a potent disease modifying therapy whose effects at MTD on TCD velocities and stroke risk has not been determined within Africa. We designed the Stroke Prevention with Hydroxyurea Enabled through Research and Education (SPHERE) trial to estimate the stroke risk among Tanzanian children using TCD screening and to determine the effectiveness of hydroxyurea at MTD to prevent stroke in this population.
Methods: SPHERE (NCT03948867) is a single center prospective phase 2 open-label trial at Bugando Medical Centre in Mwanza, Tanzania. Local personnel received hands-on TCD training with a super-examiner, and completed high-quality exams independently before formal certification. Children with SCA age 2-16 years old were enrolled; previous clinical stroke was an exclusion criterion while recent febrile illness, red cell transfusion, or hospitalization were temporary exclusions. Study participants with maximum Time-Averaged Mean Velocity (TAMV) on TCD exam categorized as conditional (170-199 cm/sec) or abnormal (≥200 cm/sec) were offered hydroxyurea with escalation to MTD, while those with normal TCD screening exams were rescreened annually. Hydroxyurea commenced at ~20 mg/kg/day using 500 mg capsules, then escalated every 8 weeks by 5 mg/kg/day to a maximum of 35 mg/kg/day based on lab parameters. Children were seen monthly during dose escalation and quarterly after reaching MTD. The primary study endpoint was change in TCD velocity after 12 months of hydroxyurea.
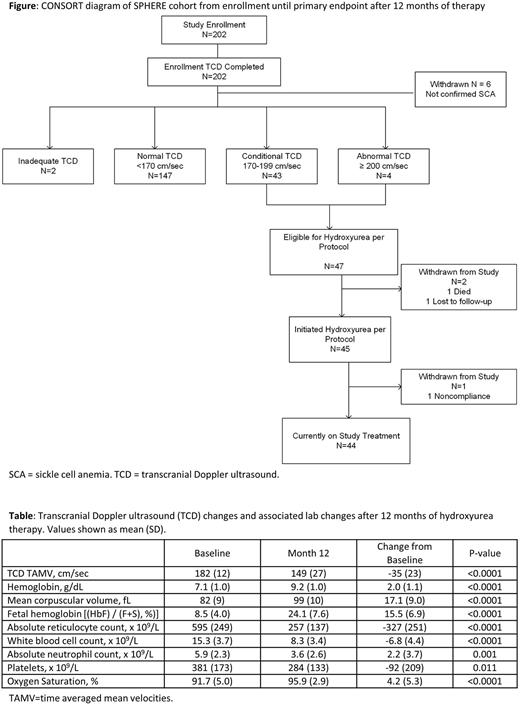
Results: From April 2019 to April 2020, a total of 202 children underwent TCD screening; 196 with confirmed SCA were included in intention-to-treat analysis. The average age (mean ± SD) at enrollment was 6.8±3.5 years, and 53% were female. Most children had documented clinical severity including painful vaso-occlusive episodes (93%), malaria (88%), transfusion (68%), and hospitalization (91% overall, 30% with >5 hospitalizations). Baseline labs included hemoglobin = 7.8±1.3 g/dL, hemoglobin F (HbF) = 9.3±5.4 %, and ANC = 5.5±2.4 x 109/L. TCD at enrollment included 75% normal, 24% elevated (22% conditional, 2% abnormal), and 1% inadequate exams. The average TAMV was 138±18 cm/sec (median 140, IQR 129-151) for 147 children in the normal category, and 182±12 cm/sec (median 178, IQR 174-188) for 47 children with elevated velocities. A total of 45 successfully initiated hydroxyurea treatment, as 1 withdrew and 1 died before treatment initiation; 1 withdrew after hydroxyurea initiation due to nonadherence. The starting dose was 20.2±1.4 mg/kg/day and escalated to 27.4±5.1 mg/kg/day at Month 12. Hemoglobin increased to 9.2±1.0 g/dL, HbF rose to 24.1 ± 7.6 %, and ANC declined to 3.6 ± 2.6 x 109/L. The 12-month TCD was obtained in 42/44 children with average TCD velocity of 149±27 cm/sec, with an average decline of 35±23 cm/sec. The stroke risk category improved from 43 conditional and 2 abnormal at baseline to 35 normal, 6 conditional and 1 abnormal at Month 12. Higher hemoglobin, higher oxygen saturation, and lower ANC at 12 months correlated with a greater reduction in TCD value, but none were statistically significant. Adverse events during 12 months of therapy were primarily severity grade 2, and no clinical strokes were recorded.
Conclusions: SPHERE has successfully documented (1) building local capacity with clinical training, research infrastructure resources, and high-quality TCD performance is feasible in Tanzania; (2) children with SCA in Tanzania have a high baseline risk for primary stroke; (3) hydroxyurea treatment starting with a moderate dose and prompt escalation to MTD significantly lowers TCD velocities; and (4) hydroxyurea at MTD reduces the primary stroke risk. As hydroxyurea access expands to more children with SCA in Tanzania, the SPHERE results indicate that TCD screening and hydroxyurea treatment at MTD can maximize stroke prevention strategies.
Disclosures
Ware:BMS: Research Funding; Addmedica: Research Funding; Hemex Health: Research Funding; Nova Laboratories: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: DSMB Chair; Editas: Other: DSMB Chair.
Author notes
Asterisk with author names denotes non-ASH members.