Abstract
INTRODUCTION: While the majority of patients with early-stage (ES), bulky Hodgkin lymphoma (HL) can be cured with modern approaches, approximately 15% of patients with bulky HL relapse. Improving upfront approaches while minimizing long-term adverse effects remains integral. We previously reported the results of a multicenter pilot study of 4 cycles of brentuximab vedotin (BV) and doxorubicin, vinblastine, and dacarbazine (AVD) followed by 1 of 4 approaches if positron emission tomography (PET)-4 negative. The treatment program was highly active and well-tolerated. We now present extended results and prognostic evaluation of baseline metabolic tumor volume (MTV) and PET2 results.
METHODS: Full methodology has been published (Kumar et al., J. Clin Oncol. 2021). Briefly, patients with untreated HL were enrolled into 1 of 4 sequential cohorts. In cohort 1 (C1), eligible patients had any of the following unfavorable (UF) risk factors: bulky mediastinal mass, ESR ≥ 50 mm/h or ≥ 30 mm/h if B symptoms, extranodal involvement, > 2 lymph node sites, or infradiphragmatic disease. The same criteria applied to C2, except for bulk being defined by the Memorial Sloan Kettering (MSK) definition (maximal transverse or coronal diameter of the largest mass > 7 cm). In C3 and C4, all patients were required to have MSK criteria bulk. All cohorts received BV-AVD x 4. Patients with a PET-negative response after 4 cycles received 30-Gy involved site radiation therapy (ISRT) in C1, 20-Gy ISRT in C2, 30-Gy consolidative-volume RT (CVRT) in C3, and no RT in C4. For 84 patients at MSK, baseline MTV was measured using the 41% maximum standard uptake value (SUV) threshold method. Interim PET after 2 cycles was interpreted using the 5-point scale with scores ≤ 3 considered negative. Survival data was updated through July 1, 2022. Progression-free (PFS) and overall survival (OS) probabilities were estimated by the Kaplan-Meier method and compared for various features using the log-rank test, with a p-value of ≤ 0.05 statistically significant.
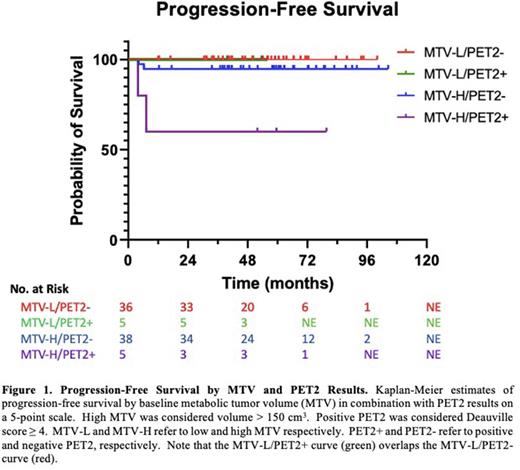
RESULTS: Of 117 patients enrolled, 116 completed chemotherapy. With a median followup of 4.6 years (6.7, 5.3, 4.5, and 3.3 years for C1-4), there were 8 progression events and 2 deaths. Since original publication, 1 further progression occurred amongst a patient in C4. The median PFS and OS have not been reached in either group. The overall 4-year PFS and OS were 93% and 98%, respectively. The 4-year PFS for C1-4 was 93%, 96%, 90%, and 93%, respectively (p=0.78). Next, outcomes were compared by MTV and PET2. Among 84 patients assessed for baseline MTV, median MTV was 150 (range 13-827) cm3. Using the median MTV to stratify patients, overall 4-year PFS in the MTV-high (n=43) versus MTV-low (n=41) group was 91% versus 100%, respectively (p=0.047). Fourteen patients (12%) had positive PET2. Four-year PFS in the PET2 positive versus negative group was 79% versus 95%, respectively (p=0.02). By MTV and PET2, 4-year PFS for the MTV-low/PET2-negative (n=36), MTV-low/PET2-positive (n=5), MTV-high/PET2-negative (n=38), and MTV-high/PET2-positive (n=5) groups was 100%, 100%, 95%, and 60%, respectively (p=0.001) (Figure 1). Within these 4 groups, there were 4 progression events, 2 within the MTV-high/PET2-negative group and 2 within the MTV-high/PET2-positive group. The 2 patients in the MTV-high/PET2-negative group had Deauville score (DS) 2 on PET2 and then developed DS4 on PET4; 1 received salvage R-ICE (concomitant CD20 expression) followed by autologous transplant (autoSCT), and 1 received salvage pembrolizumab-GVD followed by autoSCT. The 2 patients within the MTV-high/PET2-positive group had DS4 on PET2 and PET4. One patient received salvage augmented ICE followed by radiation and autoSCT. The other patient had a negative biopsy of PET4 disease and completed CVRT, but relapsed 2 months later and received salvage augmented ICE and autoSCT. All 4 of these patients remain without disease after a median follow-up post autoSCT of 65 (28-86) months.
CONCLUSIONS: We report extended followup of short-course BV-AVD followed by various doses/fields of RT, including no RT, in ES, bulky HL. The BV-AVD program remains highly active and well-tolerated, including in the absence of consolidative RT for certain patients. Baseline MTV and PET2 identifies an unfavorable cohort at higher risk of treatment failure. Trials in ES, bulky HL should evaluate stratified treatment paradigms using baseline MTV and PET2.
Disclosures
Casulo:Gilead: Research Funding; Genentech: Research Funding; Verastem: Research Funding; Bristol Myers Squibb: Research Funding; Secura Bio: Research Funding. Advani:ADC Therapeutics, Cyteir, Daiichi Sankyo, Gilead, Merck, Regeneron, Roche, Seattle Genetics: Research Funding; ADC Therapeutics, BMS, Daiichi Sankyo, Epizyme, Gilead, Incyte, Merck, Roche, Sanofi: Consultancy. Budde:Genentech: Other: Advisory Board; ADC Therapeutics: Other: Advisory Board; Merck: Research Funding; AstraZeneca: Research Funding; Amgen Inc: Research Funding; Ziopharm Oncology Inc: Other: DMSC member for a phase 1 clinical trial. Barr:Merck, abbive, gilead, Beigene, Genentech, Astrazeneca, Janssen, TG therapeutics, Celgene, BMS, Morphosys, Adaptive: Consultancy. Batlevi:Epizyme: Research Funding; Dava Oncology: Other: Provision of Services; Seattle Genetics: Consultancy; Juno/Celgene: Consultancy; Kite Pharma: Consultancy; Life Sciences: Consultancy; ADC Therapeutics: Other: Provision of Services; Janssen: Research Funding; Bristol-Myers Squibb: Other: Ownership / Equity Interests; Provision of Services; Autolus: Research Funding; Bayer: Research Funding; Xynomic: Research Funding; Novartis: Research Funding; Roche/Genentech: Research Funding; GLG Pharma: Consultancy. Horwitz:ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics,: Research Funding; C4: Research Funding; Affimed: Research Funding; Cimieo Therapeutics: Honoraria; Takeda: Consultancy; Yingli Pharma Limited and Tubulis: Honoraria; Kyowa Hakko Kirin: Consultancy; Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; SecuraBio: Honoraria; ADC Therapeutics: Research Funding; Millennium /Takeda: Research Funding; Verastem/SecuraBio: Research Funding; Crispr Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Kyowa Hakko Kirin: Research Funding; Celgene: Research Funding; Affimed,: Consultancy. Matasar:Bayer: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy; F. Hoffmann-La Roche Ltd.: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Current equity holder in private company; IMV Therapeutics: Consultancy, Honoraria; Janssen: Honoraria, Research Funding; IGM Biosciences: Research Funding; TG Therapeutics: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy, Honoraria; Pharmacyclics: Honoraria, Research Funding; ImmunoVaccine Technologies: Honoraria, Research Funding; Teva: Consultancy; GlaxoSmithKline: Honoraria, Research Funding; Karyopharm: Consultancy; Epizyme: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Research Funding; Rocket Medical: Consultancy, Research Funding. Noy:Janssen: Research Funding. Palomba:BeiGene: Consultancy; Ceramedix: Consultancy. Straus:Takeda Pharmaceuticals: Consultancy; Seagen: Consultancy. Younes:Astrazeneca: Current Employment. Zelenetz:Beigene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pharmacyclics/Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead/Kite Pharma: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria, Research Funding; Juno Pharmaceuticals: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; MBS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Moskowitz:Merck: Honoraria. Kumar:BridgeBio Pharmaceuticals: Current equity holder in publicly-traded company; Abbvie: Research Funding; Adaptive Biotechnologies: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding; AstraZeneca: Honoraria, Research Funding; Kite Pharma: Honoraria; Janssen: Honoraria. Moskowitz:ADC Therapeutics: Research Funding; Biegene: Research Funding; Miragen: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding; Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; SecuraBio: Research Funding; Affimed: Honoraria; Imbrium Therapeutics L.P./Purdue: Honoraria; Janpix Ltd: Honoraria; Merck: Honoraria; Seattle Genetics: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.