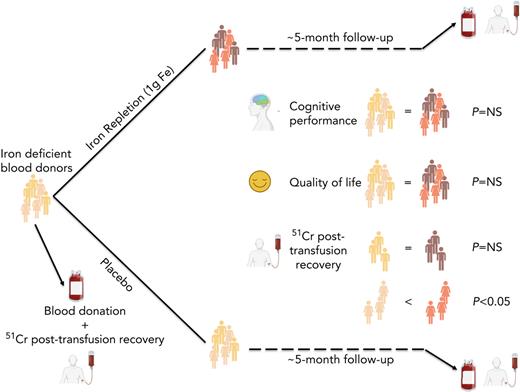
Key Points
Blood donation–induced iron deficiency did not significantly affect red blood cell quality for transfusion.
Iron repletion of iron-deficient blood donors did not improve measures of quality of life or cognition.
Abstract
Although altruistic regular blood donors are vital for the blood supply, many become iron deficient from donation-induced iron loss. The effects of blood donation–induced iron deficiency on red cell transfusion quality or donor cognition are unknown. In this double-blind, randomized trial, adult iron-deficient blood donors (n = 79; ferritin < 15 μg/L and zinc protoporphyrin >60 μMol/mol heme) who met donation qualifications were enrolled. A first standard blood donation was followed by the gold-standard measure for red cell storage quality: a 51-chromium posttransfusion red cell recovery study. Donors were then randomized to intravenous iron repletion (1 g low-molecular-weight iron dextran) or placebo. A second donation ∼5 months later was followed by another recovery study. Primary outcome was the within-subject change in posttransfusion recovery. The primary outcome measure of an ancillary study reported here was the National Institutes of Health Toolbox–derived uncorrected standard Cognition Fluid Composite Score. Overall, 983 donors were screened; 110 were iron-deficient, and of these, 39 were randomized to iron repletion and 40 to placebo. Red cell storage quality was unchanged by iron repletion: mean change in posttransfusion recovery was 1.6% (95% confidence interval −0.5 to 3.8) and −0.4% (−2.0 to 1.2) with and without iron, respectively. Iron repletion did not affect any cognition or well-being measures. These data provide evidence that current criteria for blood donation preserve red cell transfusion quality for the recipient and protect adult donors from measurable effects of blood donation–induced iron deficiency on cognition. This trial was registered at www.clinicaltrials.gov as NCT02889133 and NCT02990559.
Introduction
Each year, ∼5 million regular donors provide most of the blood supply for the United States by voluntarily donating blood.1 Despite meeting all Food and Drug Administration (FDA) requirements for blood donation, iron deficiency develops in ∼35% of regular donors after iron loss from their repeated donations.2 Concerns have been expressed for more than half a century about whether iron deficiency affects donor health or the quality of the donated blood that is transfused into the recipient. The Association for the Advancement of Blood & Biotherapies established an Ad Hoc Iron-Deficiency Working Group, which recommended that measures be adopted to identify and prevent iron deficiency in all, or selected, high-risk individuals.3 However, these recommendations were based on expert opinion because of a lack of available definitive study results.
The key regulatory measure of red cell quality for transfusion, the 51-chromium posttransfusion recovery study, requires that, at outdate (i.e., at 42 days of refrigerated storage for most red cell products), using healthy volunteers, more than 75% of transfused 51-chromium-labeled red cells, on average, circulate for 24 hours.4-6 In several older studies,7-9 red cells obtained from donors with iron-deficiency anemia were transfused into healthy recipients, without prior refrigerated storage. These studies suggested that iron-deficient red cells harbor an intrinsic defect leading to enhanced clearance from the circulation. In addition, in an animal blood banking and transfusion model, the posttransfusion recovery of red cells obtained from iron-deficient donors was significantly reduced and would not meet regulatory criteria for blood donation.10 Clinically, poorer red cell posttransfusion recovery results in extravascular hemolysis and failure of the cleared red cells to achieve their intended therapeutic purpose of optimally delivering oxygen to the recipient’s tissues.11-13
With respect to the blood donor, the physiologic consequences of blood donation–induced iron deficiency in humans have not been rigorously examined, despite evidence for adverse effects of nutritional iron deficiency on human quality of life and cognition.14 A pragmatic, randomized trial of 45 263 whole blood donors showed that increased donation frequency increased donation-related symptoms, such as fatigue, and decreased both hemoglobin and serum ferritin concentrations but did not affect secondary measures of quality of life, physical activity, or cognitive function.15 Furthermore, in a randomized trial of iron-deficient nonanemic adult blood donors, intravenous iron repletion did not improve fatigue or secondary measures of quality of life.16
Herein, we report the results of a single-center, randomized, double-blind, placebo-controlled trial (Donor Iron Deficiency Study [DIDS]) examining the effect of iron repletion on the transfusion quality of red cells obtained from adult donors who met all regulatory criteria for blood donation but were iron deficient. Younger donors, 16 to 18 years old, were excluded.17 Secondary outcomes examining quality of life were also evaluated. We also report the main outcomes of an ancillary study examining the effect of iron repletion on cognitive function. We hypothesized that stored red cells from adult donors with iron deficiency would fail to meet quality criteria and that iron repletion would improve posttransfusion recovery. In addition, we hypothesized that iron repletion of these donors would improve quality of life and cognitive performance measures.
Methods
Trial design and participants
DIDS was an investigator-initiated, prospective, randomized, double-blind, placebo-controlled trial. Details of the trial’s objectives, design, and recruitment were published (see the supplemental Appendix on the Blood website for study protocol and statistical analysis plan).17 Frequent blood donors (male ≥ 2 and female ≥ 1 whole blood donations in the past year) were recruited by email sent by the New York Blood Center and then invited for a laboratory screening visit at Columbia University Irving Medical Center in New York, New York. Trial coordinators performed an initial telephone screening of referred blood donors, who were subsequently assessed by laboratory testing for inclusion or exclusion. Eligible volunteers were 18 to 75 years old, healthy by self-report, with hematocrit levels meeting blood donation criteria (male > 39%; female > 38%) and evidence of iron deficiency (ferritin < 15 μg/L and zinc protoporphyrin > 60 μMol/mol heme). Main exclusion criteria were ineligibility for blood donation based on the New York Blood Center autologous donor questionnaire, blood donor vital sign criteria, C-reactive protein >10 mg/L, pregnancy, history of severe asthma requiring hospitalization, allergic eczema (atopic dermatitis), or other atopic allergy associated with anaphylaxis. All subjects signed informed consent forms before the initial laboratory screening was performed. Institutional ethics boards at both Columbia University Irving Medical Center and the New York Blood Center approved the research protocol.
Randomization and blinding
Study subjects were randomized using a computerized system with equal allocation (1:1) to iron repletion (intravenous 1 g low-molecular-weight iron dextran) or placebo (intravenous saline) using randomly permuted block sizes. Randomization was stratified by sex to ensure that this key characteristic was balanced between treatment groups. The randomization scheme was generated by an independent statistician in advance of the study and provided to the research pharmacy, where blinding occurred. Thus, the research pharmacist provided the placebo (intravenous saline) or treatment (intravenous iron) in tinted infusion bags with tubing specifically designed to maintain blinding in clinical research studies (Medipak). A research nurse unaffiliated with the study team was responsible for the test infusion and total dose iron infusion. Subjects in both groups received similar premedication and similar discharge instructions as if they had received low-molecular-weight iron dextran. Scheduling and logistic communications were performed by blinded study coordinators. Furthermore, all laboratory testing and outcome measures were evaluated by blinded individuals.
Procedures
Donors were recruited between January 17, 2017, and January 29, 2021, with a final 5-month follow-up on October 5, 2021. Subjects donated an initial standard autologous, leuko-reduced whole blood unit, which was refrigerated in Additive Solution Formula 3 (Haemonetics) for 40 to 42 days, and then followed by the key reference standard for blood quality: a 51-chromium posttransfusion recovery study based on methods in Moroff et al.4 Subjects were then randomized within 30 days to either intravenous normal saline (500 mL) or 1 g low-molecular-weight iron dextran (INFeD; Patheon Italia S.p.A.). All subjects received intravenous methylprednisolone (125 mg) both before and after infusion and oral acetaminophen (650 mg) and diphenhydramine (25 mg) once before infusion. Due to supply chain constraints, the original protocol was amended, and 1 subject received 1 g ferric carboxymaltose (Injectafer; Vifor (International) Inc) instead of INFeD, in similar fashion. Subjects were scheduled 150 ± 30 days following randomization for a second autologous blood donation, followed 40 to 42 days later by a second 51-chromium posttransfusion recovery study. Safety quality-of-life surveys and cognitive function were assessed immediately prior to both blood donations and both posttransfusion recovery studies (ie, twice before randomization and twice thereafter).
Outcomes
The primary outcome measure was the within-subject change in posttransfusion recovery from the first study under conditions of iron deficiency and the second posttransfusion study after randomization to iron repletion or placebo. All secondary outcomes were assessed on both blood donation and posttransfusion recovery study days, including laboratory measures of iron deficiency (ie, hemoglobin, hematocrit, zinc protoporphyrin, reticulocyte hemoglobin, ferritin, transferrin saturation, soluble transferrin receptor, hepcidin). Hepcidin was measured using an enzyme-linked immunosorbent assay kit following the manufacturer’s instructions (Intrinsic LifeSciences). All other laboratory tests were performed using clinically validated instruments in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. Printed surveys assessing health status and quality of life were also self-administered. These included the RAND Short Form 36 Health Survey version 1.0,18 which assesses 8 health concepts (ie, physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy or fatigue, and general health perceptions), each scored on a 0% to 100% scale with higher scores representing improved health or quality of life. The Multidimensional Assessment of Fatigue Global Fatigue Index,19 Beck Depression Inventory-II,20 Beck Anxiety Inventory,21 and Restless Leg Syndrome Rating Scale22 were also administered; higher scores represent decreased well-being or quality of life.
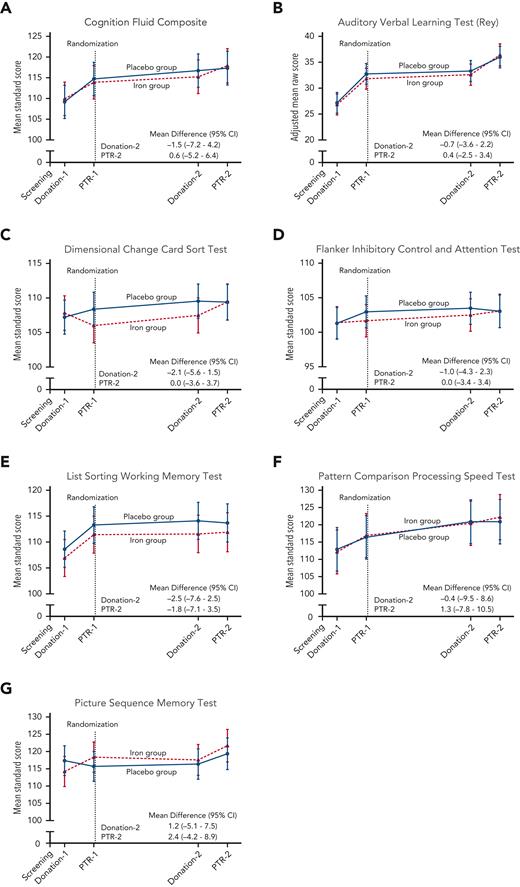
An ancillary study assessed cognitive performance in these subjects; the prespecified primary outcome was the National Institutes of Health Toolbox for Assessment of Neurological and Behavioral Function–derived uncorrected standard Cognition Fluid Composite Score.23 Secondary outcomes were uncorrected standard scores on the individual test instruments, including the Dimensional Change Card Sort Test (Executive Function-Cognitive Flexibility), the Flanker Inhibitory Control and Attention Test (Executive Function-Inhibitory Control and Attention), the Picture Sequence Memory Test (Episodic Memory), the List Sorting Working Memory Test (Working Memory), and the Pattern Comparison Processing Speed Test (Processing Speed). Raw scores on the Rey Auditory Verbal Learning Test were also assessed. The battery of tests was administered via a touch screen (Apple iPad), in person by a coordinator; the latter was trained and competency assessed in test administration by a neuropsychologist (E.C.).
Statistical analysis
A prespecified statistical analysis plan was followed (see the supplemental Appendix). The null hypothesis was that there would be no significant between-group difference in the mean within-subject change in posttransfusion recovery from the first study under conditions of iron deficiency and the second posttransfusion recovery study after randomization to iron repletion or placebo. The primary null hypothesis was tested in an intent-to-treat analysis using a t test. A sample size of 26 evaluable subjects in each trial group was calculated for the primary outcome on the basis of an alpha level of .05, a beta level of .80, a standard deviation (SD) of 5, and a minimal clinically important difference of 4% in posttransfusion recovery.17 Prespecified demographic variables (sex [male or female], race [White or non-White], age [<50 or ≥50 years]) were also explored.
The secondary and ancillary primary outcome null hypotheses were tested in an intent-to-treat analysis using linear mixed models for repeated measures to compare differences in the iron repletion and placebo group temporal course at the 4 defined time points. Subjects did not need to complete both posttransfusion recovery outcome measures to be included in secondary and ancillary study analyses. Two interim analyses were performed (after every 20 subjects completed study participation) in addition to the final analysis. No multiple comparison adjustments for the secondary and ancillary end points were defined. Therefore, only point estimates and 95% confidence intervals (CIs) are provided. The CIs were not adjusted for multiple comparisons and should not be used to infer definitive treatment effects. SAS Studio version 3.8 (SAS Institute Inc) was used for all analysis and Prism version 9.3 (GraphPad) was used for making figures.
Role of the funding source
The trial was funded by the National Heart, Lung, and Blood Institute with no industry or sponsor involvement. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. All authors provided approval to submit the manuscript for publication.
Results
Patients
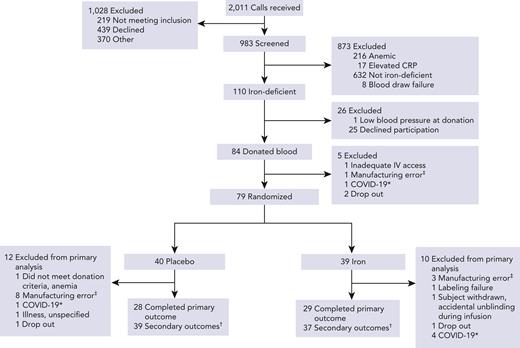
Of 2011 blood donors screened, 983 met the eligibility criteria, as determined by the initial telephone interview, and agreed to laboratory test screening; of these, 110 met the laboratory criteria for iron deficiency (Figure 1). In all, 79 subjects underwent randomization, with 39 assigned to iron repletion and 40 to placebo. For the primary outcome measure, 29 subjects (74%) in the iron repletion group and 28 subjects (70%) in the placebo group had both scheduled posttransfusion recovery studies completed successfully. Secondary outcomes were evaluable in 37 subjects (95%) in the iron repletion group and 39 subjects (98%) in the placebo group.
Consort diagram for participant flow by treatment arm. ∗Due to pandemic-related restrictions for human subject research these participants were withdrawn. †Participants who completed at least 1 cognitive performance evaluation after randomization. ‡Manufacturing errors included collection in storage solutions other than Additive Solution Formula 3, overfilled units, and otherwise compromised units. CRP, C-reactive protein; IV, intravenous.
Consort diagram for participant flow by treatment arm. ∗Due to pandemic-related restrictions for human subject research these participants were withdrawn. †Participants who completed at least 1 cognitive performance evaluation after randomization. ‡Manufacturing errors included collection in storage solutions other than Additive Solution Formula 3, overfilled units, and otherwise compromised units. CRP, C-reactive protein; IV, intravenous.
The baseline demographic data in Table 1 show no significant between-group differences for the iron repletion and placebo groups. The characteristics of the subjects who did not complete the primary outcome measure were similar to those of the remaining cohort, except that slightly more White non-Hispanic subjects did not complete the primary outcome measure (supplemental Table 1).
Laboratory outcomes were assessed at screening, first donation, and first posttransfusion recovery study, which occurred prior to randomization, and then at a second donation and posttransfusion recovery study, which occurred after randomization. The first donation occurred a median 21 days (interquartile range [IQR] 14-28) after screening, followed by the first posttransfusion recovery study 40 to 42 days later. The randomization visit occurred a median 1 day (1-1) later, followed by the second blood donation a median 145 days (129-162) afterward. Due to pandemic-related logistical restrictions, 6 subjects deviated from the protocol and the second blood donation occurred 192 to 392 days after randomization. In all cases, the second posttransfusion recovery study followed the second donation by 40 to 42 days.
Laboratory outcomes
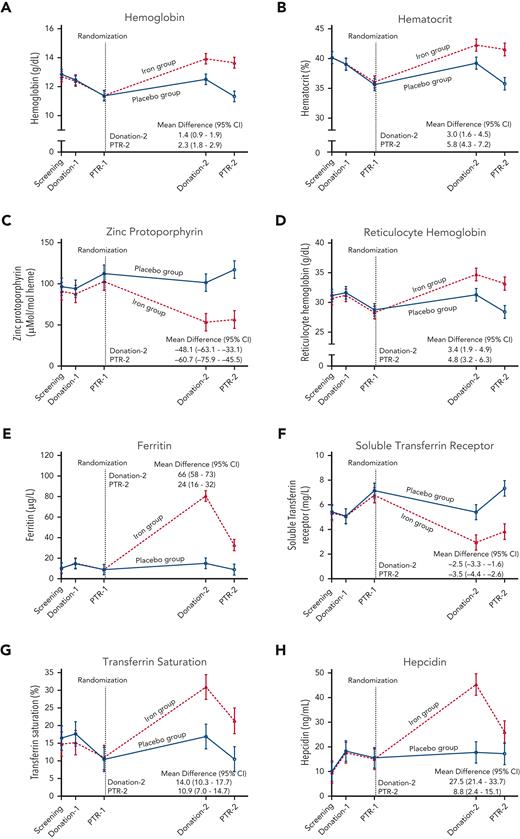
Laboratory measures of iron deficiency did not differ between the groups at any time point prior to randomization (Figure 2, supplemental Table 2). At the second blood donation visit 4 to 6 months after randomization, hemoglobin increased by a mean 1.4 g/dL (95% CI 0.9-1.9), ferritin increased by 66 μg/L (58-73), and zinc protoporphyrin decreased by 48.1 μMol/mol heme (33.1-63.1) in the iron repletion group, as compared with the placebo group. Laboratory measures by sex, age, and race subgroups are shown in the appendix (supplemental Tables 3-5). In post hoc analyses, at the donation ∼5 months after randomization, female subjects in the placebo group would more likely be deferred from allogeneic donation by being unable to meet minimum hemoglobin and hematocrit FDA criteria, as compared with those receiving iron repletion (supplemental Table 6). Furthermore, the mean hemoglobin concentration in the blood product units donated after randomization was 18.7 g/dL (95% CI 18.4-19.1) in the iron repletion group and 17.5 g/L (17.0-18.0) in the placebo group (supplemental Figure 1, supplemental Table 7).
Laboratory measurements during the trial. The data points represent the estimated means based on a mixed-model repeated-measures analysis after adjustment for the baseline value. The vertical bars denote 95% CIs. The dependent variable was the laboratory value at each predetermined time point. Fixed effects included the interaction between treatment and time. Time was treated as a categorical variable. The subject was included in the model as a random effect. A first-order autoregressive covariance matrix was used to model the within-patient variance-covariance errors. Prespecified secondary outcomes were laboratory measures of (A) hemoglobin, (B) hematocrit, (C) zinc protoporphyrin, (D) reticulocyte hemoglobin, (E) ferritin, (F) soluble transferrin receptor, (G) transferrin saturation, and (H) hepcidin. PTR, posttransfusion recovery.
Laboratory measurements during the trial. The data points represent the estimated means based on a mixed-model repeated-measures analysis after adjustment for the baseline value. The vertical bars denote 95% CIs. The dependent variable was the laboratory value at each predetermined time point. Fixed effects included the interaction between treatment and time. Time was treated as a categorical variable. The subject was included in the model as a random effect. A first-order autoregressive covariance matrix was used to model the within-patient variance-covariance errors. Prespecified secondary outcomes were laboratory measures of (A) hemoglobin, (B) hematocrit, (C) zinc protoporphyrin, (D) reticulocyte hemoglobin, (E) ferritin, (F) soluble transferrin receptor, (G) transferrin saturation, and (H) hepcidin. PTR, posttransfusion recovery.
Primary outcome
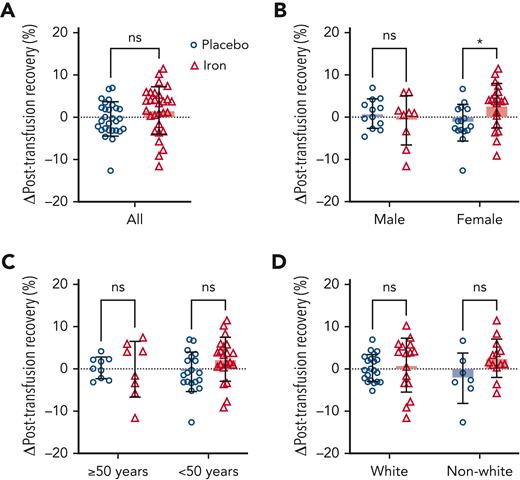
FDA criteria require that, at the end of the maximal allowable storage duration (42 days), the posttransfusion recovery should be at least 75% with a sample SD not to exceed 9%. In addition, the lower limit of the 95% CI for the proportion of the population that have recovery of at least 75% must be ≥70%. By these criteria, our subjects with iron deficiency met the FDA criteria with a mean (±SD) recovery of 83.0% ± 6.5% and 71 of 76 successful recoveries (ie, based on all subjects prior to randomization). The primary outcome mean increase in posttransfusion recovery among subjects randomized to iron repletion was 1.6% (95% CI −0.5 to 3.8) as compared with −0.4% (−2.0 to 1.2) among those randomized to placebo, for a nonsignificant mean between-group difference of 2.0% (−0.6 to 4.6) (Figure 3, supplemental Table 8). Prespecified subgroup analysis by sex (Figure 3), age, and race are shown in the supplemental Appendix (supplemental Table 8). The mean between-group difference in female and male subjects was 4.0% (0.7-7.4) and −1.6% (−5.9 to 2.7), respectively. In a post hoc analysis, the mean between-group difference was greatest in female subjects less than 50 years old: 4.9% (0.9-8.9; supplemental Figure 2).
Change in red blood cell posttransfusion recovery between randomization arms and by prespecified subgroups. Bars represent mean change in red blood cell posttransfusion recovery between the second measure performed after randomization and the first measure before randomization to placebo (open blue circles) or iron repletion (open red triangles). (A) Overall change in posttransfusion recovery among all randomized participants completing the primary outcome measure and by (B) sex, (C) age, and (D) race. Error bars represent SD. ∗P < .05 by unpaired t test. ns, not significant.
Change in red blood cell posttransfusion recovery between randomization arms and by prespecified subgroups. Bars represent mean change in red blood cell posttransfusion recovery between the second measure performed after randomization and the first measure before randomization to placebo (open blue circles) or iron repletion (open red triangles). (A) Overall change in posttransfusion recovery among all randomized participants completing the primary outcome measure and by (B) sex, (C) age, and (D) race. Error bars represent SD. ∗P < .05 by unpaired t test. ns, not significant.
Quality of life and cognitive performance outcomes
Mean scores on quality-of-life measures (ie, Beck Anxiety Inventory, Beck Depression Inventory II, Global Fatigue Index, Restless Leg Syndrome Rating Scale, and each component of the Short Form 36 Health Survey) were not significantly affected by iron repletion at any time point (supplemental Figures 3, 4). In an ancillary study examining cognition using the National Institutes of Health Toolbox for Assessment of Neurological and Behavioral Function in the same subjects, the ancillary primary outcome cognition fluid composite standard score measure did not significantly differ between groups (Figure 4). Mean scores for each component measure also did not significantly differ between groups at any time before or after randomization. Despite inducing anemia in most donors (Figure 2, supplemental Table 2), blood donations did not significantly affect any of the cognitive or quality-of-life outcomes measured at 40 to 42 days after donation and immediately before each posttransfusion recovery study.
Cognitive performance measurements during the trial. The data points represent the estimated means based on a mixed-model repeated-measures analysis after adjustment for the baseline value. The vertical bars denote 95% CIs. The dependent variable was the cognitive test score at each predetermined time point. Fixed effects included the interaction between treatment and time. Time was treated as a categorical variable. The subject was included in the model as a random effect. A first-order autoregressive covariance matrix was used to model the within-patient variance-covariance errors. The primary outcome was the (A) Cognition Fluid Composite Score. Prespecified secondary outcomes were the (B) raw score on the Auditory Verbal Learning Test (Rey), (C) Dimensional Change Card Sort Test, (D) Flanker Inhibitory Control and Attention Test, (E) List Sorting Working Memory Test, (F) Pattern Comparison Processing Speed Test, and (G) Picture Sequence Memory Test. Standard scores have a mean of 100 and SD of 15, with higher scores representing better cognitive performance. PTR, posttransfusion recovery.
Cognitive performance measurements during the trial. The data points represent the estimated means based on a mixed-model repeated-measures analysis after adjustment for the baseline value. The vertical bars denote 95% CIs. The dependent variable was the cognitive test score at each predetermined time point. Fixed effects included the interaction between treatment and time. Time was treated as a categorical variable. The subject was included in the model as a random effect. A first-order autoregressive covariance matrix was used to model the within-patient variance-covariance errors. The primary outcome was the (A) Cognition Fluid Composite Score. Prespecified secondary outcomes were the (B) raw score on the Auditory Verbal Learning Test (Rey), (C) Dimensional Change Card Sort Test, (D) Flanker Inhibitory Control and Attention Test, (E) List Sorting Working Memory Test, (F) Pattern Comparison Processing Speed Test, and (G) Picture Sequence Memory Test. Standard scores have a mean of 100 and SD of 15, with higher scores representing better cognitive performance. PTR, posttransfusion recovery.
Adverse events
The percentage of patients reporting 1 or more adverse events was similar in the 2 groups: 2.6% in the iron repletion group and 10% in the placebo group (supplemental Table 9).
Discussion
The results of this randomized, double-blind, placebo-controlled trial provide evidence that current regulatory criteria for blood donation preserve the storage quality of red cells for transfusion into recipients and protect adult donors from measurable effects of blood donation–induced iron deficiency on quality of life and cognition. DIDS examined the effects of iron repletion of adult blood donors who met all regulatory criteria for whole blood donation but were demonstrably iron deficient. Iron repletion successfully corrected iron deficiency, whereas placebo-treated donors remained iron deficient, with some developing anemia to levels below what would typically be allowed for allogeneic blood donation. Iron repletion did not significantly alter the primary outcome, the posttransfusion red cell recovery, or any of the secondary outcomes, including standard measures of quality of life and cognitive function. The incidence of adverse events also did not differ significantly between the groups. Because oral iron, the clinically preferred method for iron repletion, may cause gastrointestinal side effects that could allow identification of treatment assignment,24 we used intravenous iron to maintain double blinding. The 1-g dose of intravenous iron more than met the amount needed for the 2 scheduled blood donations because 1 U of whole blood contains ∼0.20 to 0.25 g of iron.25 Treatment efficacy is illustrated by the full recovery of hemoglobin concentrations 40 to 42 days following the second blood donation in subjects randomized to intravenous iron, whereas those receiving placebo remained largely iron deficient and anemic. These effects are similar to other published studies of iron supplementation in blood donors.26
To maintain the blood supply, red cell products are refrigerated for variable durations, which induces oxidative stress to donor red cells.27 In addition, red cells from individuals with iron-deficiency anemia may be more sensitive to this oxidative stress.28 Sex-, age-, and race-related differences could also potentially affect the susceptibility of donor red cells to storage-induced hemolysis.29-33 Furthermore, testosterone-dependent sex differences affected red cell posttransfusion recovery in a mouse model.32 Our finding, that women younger than 50 years of age, but not men or older women, have improved posttransfusion recovery following iron repletion, requires further study.
Iron deficiency is associated with various symptoms, including fatigue, depression, impaired cognition, restless leg syndrome, and diminished quality of life.14 However, despite a disproportionate frequency of iron deficiency, blood donors have not been evaluated in most prior studies. Nonetheless, in the INTERVAL trial,15 donors randomized to more frequent blood donation experienced more deferrals for low hemoglobin along with more fatigue and restless leg symptoms, but no significant differences were observed in quality of life or cognitive function. However, the lack of blinding in the INTERVAL trial may have been responsible for the increased frequency of self-reported symptoms among participants randomized to more frequent donation. In the Iron Supplementation in Blood Donors randomized controlled trial,16 iron repletion (intravenous ferric carboxymaltose 800 mg) did not induce a clinically relevant decrease in self-described fatigue and general well-being. Although secondary analyses examined participants with low ferritin levels and did not find an effect, a relatively high ferritin level (50 μg/L) was used for study inclusion in the overall study. Furthermore, treatment responses were assessed 6 to 8 weeks after iron or placebo infusion, and a longer time may be required for the effect of iron repletion to become fully apparent. Nonetheless, our findings are consistent with those of the Iron Supplementation in Blood Donors trial, along with those of a randomized controlled trial of oral iron supplementation of iron-deficient female blood donors following blood donation,34 which similarly did not observe clinically significant improvement in fatigue or quality of life.
The results of DIDS are reassuring regarding the effects of blood donation–induced iron deficiency on both adult donors and their transfusion recipients. Nonetheless, we did not examine some other potentially important consequences of blood donation–induced iron deficiency. Importantly, younger eligible donors, 16 to 18 years old, were not included, although they provided 11.2% of all donations in the United States in 2019 and have a higher risk for iron depletion.1,35 Although ongoing iron-dependent brain myelination and development in 16- to 18-year old teenagers may expose them to short- and long-term harmful effects from blood donation–induced iron deficiency,36 the existence and magnitude of these risks have not been examined rigorously. In addition, the increased risk of donor deferral and the adverse effects of temporary deferral on donor retention are well established; these decrease subsequent volunteer donations by ∼30% over a 4.25-year period.37 Indeed, in DIDS participants at blood donation after randomization to placebo, 58% of the women and 23% of the men would have been ineligible for allogeneic donation; in those randomized to iron repletion, the corresponding proportions are 16% of the women and 0% of the men. In an additional post hoc analysis, the total hemoglobin concentration from red cell units obtained from subjects randomized to iron repletion was a mean 1.2 g/dL higher than those randomized to placebo; nonetheless, other than a decreased posttransfusion hemoglobin increment,38,39 the clinical consequences to the transfusion recipient of receiving red cell units with lower hemoglobin concentrations has yet to be determined.
Because blood donors are healthier and have better self-reported quality-of-life measures (both mental and physical) than non–blood donors,40 one limitation of DIDS is its lack of generalizability to iron deficiency in non–blood donors. This selection bias may also have been exacerbated by including only blood donors who could commit the time for the multiple screening and follow-up visits that DIDS required. Our results highlight differences in iron homeostasis between donation-induced iron deficiency in blood donors and nutritional iron deficiency in non–blood donors. Blood donation acutely decreases body iron in donors whose diets generally have a high bioavailable iron content and who were previously iron replete with adequate tissue iron. Thus, with the abrupt onset of iron deficiency at donation, iron utilization for red cell production may be rapidly minimized to preserve tissue iron delivery.41 In contrast, nutritional iron deficiency results from a chronic undersupply of iron for both red cell production and tissue iron requirements and appears to adversely affect quality of life and cognition.14 Despite the large difference in body iron stores and red cell parameters achieved between the iron repletion and placebo groups, differences among subjects in dietary iron content or other environmental exposures may alter red cell redox metabolism and affect storage quality,42 thereby masking an effect of iron repletion. Finally, the posttransfusion recovery primary outcome measure only assesses the ability of transfused red cells to circulate for 24 hours. Longer-term red blood cell survival, tissue oxygenation,43 redox homeostasis,44 hemoglobin increment,38,39 or other measures of red cell quality or efficacy were not assessed and may possibly be affected by iron repletion.
In conclusion, the DIDS trial found that current regulatory criteria for blood donation maintain red cell storage quality for transfusion without exposing adult donors to measurable adverse effects on quality of life or cognition resulting from blood donation–induced iron deficiency. Nonetheless, rigorous examination is needed of the consequences of blood donation–induced iron deficiency for brain development and cognition in younger teenage donors.
Acknowledgments
The authors thank the human volunteer blood donors who took part in this study. The authors also thank the inpatient and outpatient nurses and staff of the Irving Center for Clinical and Translational Research for their outstanding patient care and support of this study; Erin Poptanich, Jane Netterwald, and Inna Gerts-Zubkov in the Center for Advanced Laboratory Medicine for laboratory support; Donna Strauss and Novelette Thomas for logistical support at the New York Blood Center; Robin Hussey and her staff for blood bank support; Jeanette Rodriguez, Rene Deknatel, and Maria Kouimanis for administrative support; Simone Glynn for her support of transfusion medicine research; and the members of the data and safety monitoring board (Katherine Ender [chair], Andrew Eisenberger, Sujit Sheth, and Mr. Carlos Delvalle).
This work was supported by National Institutes of Health grants HL133049 and HL139489 and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: E.A.H., G.M.B., E.C., C.H., Y.S., D.J.M., R.O.F., and S.L.S. designed the study and analyzed the results; Z.C.B., Y.F., J.I.G., F.L.C., L.A.S., A.T.Z., M. Soffing, A.M., J.S., C.E., M. Scotto, D.A.K., and B.H.S. helped to coordinate many crucial aspects of the trial to ensure its safe and successful operational execution; Z.C.B., Y.F., J.I.G., F.L.C., L.A.S., and A.T.Z. enrolled patients, collected data, and helped to interpret the results; M. Soffing and A.M. provided radiolabeling support and consultation for specific aspects of the trial related to nuclear medicine; C.E. and M. Scotto helped design the blinding protocol and provided research pharmacy support; E.C., C.H., and Y.S. designed and helped interpret the cognitive performance outcomes; E.A.H. and D.J.M. performed the statistical analyses for the trial; and all authors participated in the editing of the manuscript and approved the final version.
Conflicts of interest disclosure: The authors declare no competing financial interests.
Correspondence: Eldad A. Hod, Department of Pathology and Cell Biology, Laboratory of Transfusion Biology, Columbia University Irving Medical Center, 630 West 168th St, Room P&S 15-401, New York, NY 10032, USA; e-mail: eh2217@cumc.columbia.edu.
References
Author notes
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication date. Proposals for access should be sent to the corresponding author (eh2217@cumc.columbia.edu). The study protocol is included as a data supplement available with the online version of this article.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.