In this issue of Blood, Scarfò et al on behalf of the Italian Strategic Research Program on CLL present the results of the phase-II study IMPROVE, that evaluates a measurable residual disease (MRD)-driven model to individualize the treatment of relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) with discontinuation of single-agent venetoclax if displaying undetectable MRD4 (uMRD4; <10−4) or treatment intensification with addition of ibrutinib and later discontinuation of both upon uMRD4.1 The strategy was successful in 33 of 38 evaluable patients (87%) with combination therapy required in only half (16 of 33). While the combination of Bruton's tyrosine kinase (BTK) and BCL2 inhibitors is synergistic and highly effective by respectively addressing two critical survival mechanisms of CLL—inhibition of the proliferation signal from the B-cell receptor, and induction of apoptosis2-4—deep responses can be achieved with monotherapy minimizing the risk of added toxicity. IMPROVE thoughtfully addressed the challenge of identifying patients to avoid under- or overtreatment.
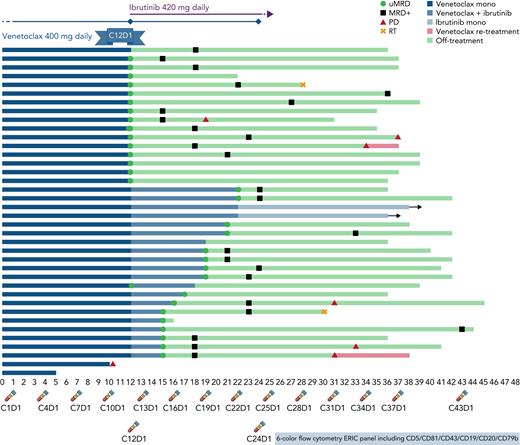
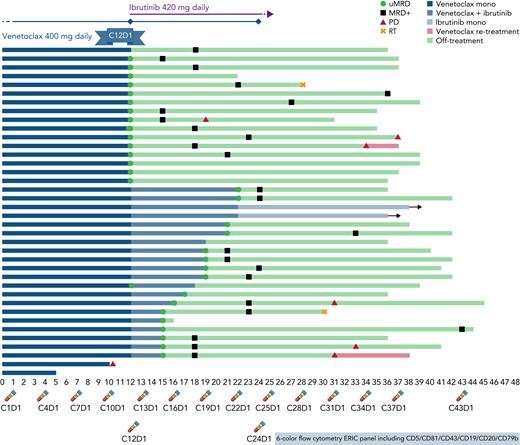
A total of 38 patients (median age 64) with R/R CLL after at least one line of therapy that did not include a BTK or BCL2 inhibitor who required therapy according to International Workshop on CLL (iwCLL) criteria were started on single-agent venetoclax at standard doses for a total of twelve 28-day cycles (see figure). This adequately reflects our current R/R CLL population, who may now be relapsing after having received chemoimmunotherapy as first line. Although BTKi’s are more commonly seen as a go-to option for monotherapy, the choice of venetoclax was based on its previously documented higher uMRD rate of up to 16% in the bone marrow (BM) in this setting,5 which is rarely achieved with ibrutinib alone.6
Strategy and outcomes. Treatment schema with initial venetoclax monotherapy followed by potential combination with ibrutinib according to MRD analysis performed every 3 months (timepoints at bottom) and swimmer’s plot with outcomes of all 38 evaluable patients.
Strategy and outcomes. Treatment schema with initial venetoclax monotherapy followed by potential combination with ibrutinib according to MRD analysis performed every 3 months (timepoints at bottom) and swimmer’s plot with outcomes of all 38 evaluable patients.
Assessment of MRD after 12 cycles of venetoclax was done with a six-color flow cytometry panel from peripheral blood (PB). Undetectable MRD4 was achieved in 19 patients and confirmed in BM in 17 (45% of intent-to-treat population). This uMRD rate is higher than previously described and may reflect a median of one previous treatment line or simply a small study sample. It is nevertheless an important finding, considering the population reflects current clinical scenarios and the high-risk features include 27/34 unmutated IGHV (79%), 7/33 del(17p) (21%), and 9/31 mutated TP53 (29%).
Ibrutinib at standard 420 mg PO daily dose was added to venetoclax on cycle 13 for the 19 patients who had not achieved uMRD4 by cycle 12 (see figure). All patients benefitted from the addition of ibrutinib. Sixteen successfully achieved PB/BM uMRD4 and were able to discontinue therapy after a median of 7 months (range 3–10). In the MURANO study (evaluating fixed venetoclax and rituximab in R/R CLL for 24 months),7 PB uMRD4 was achieved in 62% of patients during the first year without deepening of response on the second year. Curves of MRD assessment over time on IMPROVE demonstrate stable, or even rising, MRD levels in patients who did not achieve uMRD4 with venetoclax alone and clear improvement once ibrutinib was added. The authors appear to have identified a reasonable timepoint for intervention with added effect from ibrutinib in patients with suboptimal response to venetoclax.
Although iwCLL responses were evaluated with 63% of intent-to-treat patients (24 of 38) achieving uMRD4 CR with the MRD-tailored strategy, the strategy nevertheless considered only MRD-by-flow for treatment decisions. The rate of concordance between PB and BM MRD was around 90%, suggesting feasibility in standard practice where BM biopsies for CLL are rare.
After a median follow-up of 36.5 months, 10 patients had progressed for median progression free-survival not reached, and an estimated 36-month progression free-survival of 74.5%. MRD was detectable in 78% after a median of 7 months since treatment discontinuation. However, median time from MRD relapse to clinical progression was not reached, with no difference whether uMRD4 was achieved with venetoclax or combination. These are highly comparable with similar strategies that use fixed-duration combination therapies that include BTK and/or BCL2 inhibitors.
Most adverse events (AEs) were grade 1–2 with only grade 3–4 neutropenia and bronchitis occurring in more than one patient (n = 17, 43.6% and n = 2, 5%, respectively) and no discontinuation from treatment-related AE. AEs of interest for ibrutinib such as atrial fibrillation, bleeding, and hypertension all occurred in grades 1 or 2 in less than 15% of patients. It is obviously difficult to compare studies; however, these are comforting when other combination strategies of fixed-duration venetoclax–ibrutinib report similar rates of neutropenia, a frequent venetoclax complication, but higher rates of ibrutinib-related AEs, likely reflecting a longer exposure.2,8 No tumor lysis syndrome was identified despite the initial venetoclax monotherapy, and most patients with at least medium risk of tumor lysis syndrome emphasized the safety of the current venetoclax ramp-up protocol.
Patients may not only appreciate the abbreviated treatment exposure with less clinical toxicity but also lower financial toxicity. Cost-effectiveness models demonstrate the lower costs of fixed-duration therapies compared with indefinite therapies.9 In addition, the convenience of a fully oral regimen and avoidance of an anti-CD20 antibody is relevant considering its SARS-CoV-2–related complications.10
The question is where do we go from here? A large proportion of patients were able to successfully achieve deep response and interrupt therapy within approximately a year of therapy with median time to uMRD4 of 14 months (range 11–22). Can we get by with less? Median time to PB uMRD4 was 5 months (range 5–10). While median to PB/BM uMRD4 was 10 months (range 10–11), concordance between PB and MRD was around 90%. Those who required ibrutinib had in many instances passed their best MRD response from venetoclax. Earlier ibrutinib introduction may spare some months of suboptimal therapy and further shorten length without impact on disease control.
The authors provide strong proof of principle that an MRD-driven strategy is feasible in the R/R setting and there is no obvious reason why it could not be attempted in first line. Larger studies and longer follow-up should certainly follow, potentially with second generation BTK inhibitors that could further improve on the low AE rates. The time for indefinite therapy is coming to an end.
Conflict-of-interest disclosure: A.J.A. has participated in advisory committees for Amgen, Kite, SeaGen, Epizyme, Janssen, BeiGene, Incyte, and TG Therapeutics, and has received research funding from BeiGene, Incyte, and LOXO Therapeutics. C.C. declares no conflict of interest.