In this issue of Blood, Zhi et al1 describe that prophylactic administration of a human polyclonal or monoclonal anti–human platelet antigen (HPA)-1a in a preclinical and alloantigen-specific mouse model of fetal and neonatal alloimmune thrombocytopenia (FNAIT) is able to rapidly clear HPA-1a–positive platelets from the circulation, thereby preventing alloimmunization and the onset of FNAIT.
FNAIT is a rare but potentially life-threatening condition in pregnancy that can lead to bleeding complications in the fetus or neonate, including the most feared complication of intracranial hemorrhage.2,3 FNAIT results from an incompatibility between fetal and maternal HPAs, which in White subjects is most frequently HPA-1a. This leads to the development of maternal HPA immunoglobulin G-alloantibodies, which cross the placenta and bind to HPA-positive fetal or neonatal platelets, resulting in platelet destruction and thrombocytopenia in the fetus or newborn.3,4 The pathophysiology of FNAIT has similarities to that of hemolytic disease of the fetus or newborn (HDFN). HDFN develops due to an incompatibility between fetal and maternal rhesus D (RhD) and subsequent formation of maternal RhD alloantibodies that target the RhD-positive fetal or neonatal red blood cells, resulting in fetal anemia and potentially hydrops fetalis and/or death of the fetus. Striking differences between FNAIT and HDFN, apart from the source of the fetal antigen, are related to the timing of maternal alloimmunization and the management approach. In HDFN, the neonates are rarely affected in first pregnancies, and fetuses are progressively more affected in subsequent pregnancies. Conversely, FNAIT can manifest with severe clinical symptoms in first pregnancies.5 In addition, in most developed countries, there is a screening program in place for Rh incompatibility. The prophylactic administration of anti-RhD has drastically reduced the incidence of HDFN and can be seen as one of the major medical breakthroughs of the 20th century. In contrast, there is currently no antenatal screening performed for patients at risk for developing FNAIT. Consequently, FNAIT is only discovered after the first pregnancy. Thus, first-line antenatal management, consisting of weekly administration of IV immunoglobulin with or without steroids, is initiated in subsequent pregnancies.6 There are currently no clinically approved approaches for the prevention of FNAIT. Previously, it has been suggested that low doses of HPA antibodies could be effective to prevent alloimmunization and development of FNAIT.7,8
In the current study, Zhi et al report a preclinical investigation of the ability of human HPA-1a antibodies to prophylactically prevent maternal alloimmunization and development of FNAIT. They used their recently established alloantigen-specific FNAIT mouse model, which pre-immunizes wild-type female mice with platelets from transgenic mice expressing the human HPA-1a epitope on a murine glycoprotein IIIa backbone (termed APLDQ GPIIIa).9 This results in the generation of HPA-1a alloantibodies that can recapitulate FNAIT clinically when these female mice are bred with HPA-1a–positive male mice. Using this elegant alloantigen-specific FNAIT mouse model, two human HPA-1a–specific antibodies were preclinically evaluated: RLYB211 (polyclonal antibody) and RLYB212 (monoclonal antibody). It was observed that both antibodies could effectively clear HPA-1a–positive murine platelets from the circulation and prevent the occurrence of alloimmunization (determined by the presence of antibodies reactive against APLDQ platelets). Further analyses focused on RLYB211. It was first shown that a repeat administration of RLYB211 could provide sustained protection against alloimmunization to repeated immune challenges (APLDQ platelet transfusion). In addition, RLYB211 was able to elevate the platelet counts in the pups, in a manner directly related to the dose of the administered antibody.
The very encouraging results reported by Zhi et al collectively indicate that prophylactic administration of low-dose human anti–HPA-1a is able to prevent maternal alloimmunization and subsequent onset of FNAIT in an alloantigen-specific FNAIT mouse model, which provides support for use of a similar approach in humans (see figure). Limitations of the study include that the mechanism of immune suppression was not directly investigated, but it most likely involves rapid platelet-antigen clearance, preventing the development of an alloimmune response. In addition, the FNAIT mouse model used is dependent on platelet pre-immunization, as HPA-1a alloantibodies are not generated solely due to pregnancy, for yet unknown reasons. The potential for RLYB211 as a prophylactic regimen for FNAIT is further supported in a human setting by a preliminary report of 8 healthy male subjects. Administration of RLYB211 (n = 6) exhibited acceptable safety and tolerability, and it significantly accelerated the clearance of mismatched HPA-1a–positive platelets compared with placebo (n = 2).10 Overall, the road toward combining both antenatal screening and prophylaxis to combat FNAIT can be envisioned; however, large clinical trials will be needed to evaluate the safety, efficacy, and cost–benefit. In addition, implementation of antenatal screening for FNAIT will also require defined risk stratification tools.2
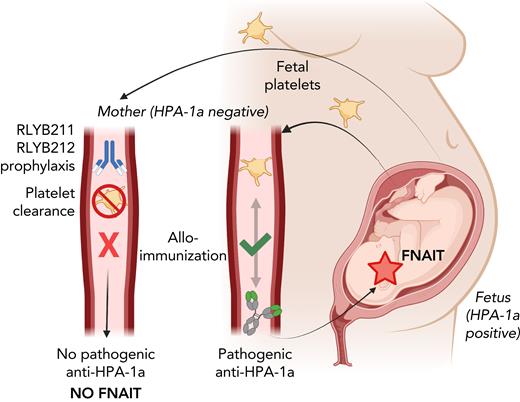
Prophylactic administration of human HPA-1a–specific antibodies protects against FNAIT in mice. In FNAIT, HPA-1a–positive fetal platelets enter the maternal circulation, resulting in maternal alloimmunization characterized by the development of pathogenic HPA-1a alloantibodies. These antibodies cross the placenta and destroy the fetal platelets, causing thrombocytopenia and bleeding symptoms in the fetus or newborn (right side). Prophylactic administration of RLYB211 (polyclonal antibody) or RLYB212 (monoclonal antibody) rapidly clears HPA-1a–positive platelets and prevents maternal alloimmunization (development of pathogenic HPA-1a alloantibodies) and subsequent onset of FNAIT in an alloantigen-specific FNAIT mouse model (left side). This provides support for use of a similar approach to prevent FNAIT in humans. Figure was created with BioRender.com.
Prophylactic administration of human HPA-1a–specific antibodies protects against FNAIT in mice. In FNAIT, HPA-1a–positive fetal platelets enter the maternal circulation, resulting in maternal alloimmunization characterized by the development of pathogenic HPA-1a alloantibodies. These antibodies cross the placenta and destroy the fetal platelets, causing thrombocytopenia and bleeding symptoms in the fetus or newborn (right side). Prophylactic administration of RLYB211 (polyclonal antibody) or RLYB212 (monoclonal antibody) rapidly clears HPA-1a–positive platelets and prevents maternal alloimmunization (development of pathogenic HPA-1a alloantibodies) and subsequent onset of FNAIT in an alloantigen-specific FNAIT mouse model (left side). This provides support for use of a similar approach to prevent FNAIT in humans. Figure was created with BioRender.com.
In summary, Zhi et al advance the field by providing proof-of-concept that prophylactic administration of monoclonal or polyclonal HPA-1a–specific antibodies may effectively prevent anti–HPA-1a alloimmunization in pregnant women at risk for FNAIT.
Conflict-of-interest disclosure: The authors declare no competing financial interests.