Abstract
Platelet transfusions are commonly administered for the prevention or treatment of bleeding in patients with acquired thrombocytopenia across a range of clinical contexts. Recent data, including randomized trials, have highlighted uncertainties in the risk-benefit balance of this therapy, which is the subject of this review. Hemovigilance systems report that platelets are the most frequently implicated component in transfusion reactions. There is considerable variation in platelet count increment after platelet transfusion, and limited evidence of efficacy for clinical outcomes, including prevention of bleeding. Bleeding events commonly occur despite the different policies for platelet transfusion prophylaxis. The underlying mechanisms of harm reported in randomized trials may be related to the role of platelets beyond hemostasis, including mediating inflammation. Research supports the implementation of a restrictive platelet transfusion policy. Research is needed to better understand the impact of platelet donation characteristics on outcomes, and to determine the optimal thresholds for platelet transfusion before invasive procedures or major surgery (eg, laparotomy). Platelet transfusion policies should move toward a risk-adapted approach that does not focus solely on platelet count.
Introduction
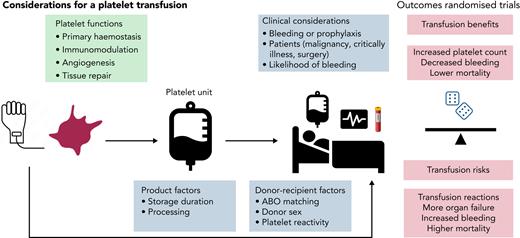
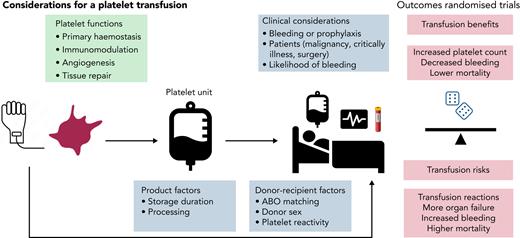
The purpose of this article is to provide a practical, evidence-based approach for the decision to administer platelet transfusion in patients with acquired thrombocytopenia. Platelet counts remain remarkably stable during life, and reference ranges for thrombocytopenia are typically defined by the lower limits: normal platelet count, 150 × 109/L; moderate thrombocytopenia, <50 × 109/L; and severe thrombocytopenia, <20 × 109/L. Approximately 100 billion platelets are produced daily by the adult bone marrow, many of which are stored in the spleen.1,2 By comparison the average yield of platelets in a platelet transfusion unit is ∼3 × 1011. Platelets are essential for primary hemostasis and maintaining vascular integrity. Therefore, treating thrombocytopenia and reducing bleeding risk with a platelet transfusion has biological plausibility, as first reported in a case study of a young man with life-threatening epistaxis in in 1910.3 Advances in processing and storage of platelet components have underpinned the expansion of platelet banking.4 Annually, >2 million platelet transfusions are administered in the United States5 and 300 000 in the United Kingdom at considerable cost, yet there are concerns about security of supply, as has been apparent during the COVID-19 pandemic.6 There is a need for a better understanding of the role of platelet transfusions for both patients (recipients) and donors.
The case studies described herein review the common underlying assumptions that drive our current platelet transfusion practice, which can be summarized as follows:
Thrombocytopenia predicts bleeding.
Platelet transfusions consistently raise platelet counts (efficacy).
Platelet transfusions prevent or treat clinical bleeding (clinical/cost effectiveness), without causing harm (safety).
How are platelet transfusions manufactured and what is in the unit?
The heterogeneity of the platelet component is often underappreciated. Platelet units are either whole blood–derived (by the buffy coat method in the United Kingdom and Canada or from platelet-rich plasma in the United States; 4 to 6 donations are pooled for an adult dose) or by apheresis (obtained from a single donor). Nearly all platelet units undergo leukocyte reduction before storage. During storage in plasma or different media, platelets undergo varying degrees of biochemical, structural, and functional changes, commonly known as the storage lesion.7,8 There is ongoing research interest into the efficacy of cold-stored platelets by comparison with standard room temperature storage.9 There is variation in platelet count between normal individuals that will affect yield after donation. Platelets from different donors exhibit many biological differences, for example the degree of responsiveness (ie, donors with highly responsive platelets have a higher level of activated platelets).10,11 In a proof-of-principle, semirandomized trial, patients with nonbleeding thrombocytopenia with myelodysplasia were randomly allocated to receive a platelet transfusion from a high- or low-response donor.12 It was hypothesized that platelets donated by a high responder would be cleared more quickly, but no differences in platelet count increments were seen after transfusion between high- and low-responder donations, although the lack of change in count may reflect the study population under evaluation and important differences may apply, for example, in patients with acute bleeding.
Other important donor/donation characteristics may affect affect recipient outcomes. Donor age and sex may affect platelet count, size, and function.13 Inflammatory cytokines may be higher in platelets from female donors.14 Because of the pressures on platelet availability and supply, substitution of platelets of a different blood group may be needed for transfusion, such that various proportions (up to a half) of all administered platelet transfusions may not be fully ABO identical.15,16 Yet, this may not represent ideal practice, and better ABO matching, however defined, may have important clinical benefits.17 Pathogen reduction technology has been applied to platelets to reduce current and future infection risks, and research is addressing safety and efficacy in different groups of recipients, and effects on platelet refractoriness.18
Case 1: hematological malignancy
A 47-year-old man with myeloma received an autologous stem cell transplant (SCT). He had experienced minor reactions to platelet transfusions and minor bleeding episodes in the past, but reported no spontaneous bleeding today. His posttransplant platelet count of 13 × 109/L yesterday was 7 × 109/L the next day. What are the factors to consider in this case when deciding on the need for a platelet transfusion?
Thrombocytopenia and risk of spontaneous bleeding
Clinicians often assume that a low platelet count predicts the risk of spontaneous (or nontraumatic) bleeding, but the strength of this relationship is unclear. In The Platelet Dose (PLADO) randomized controlled trial (RCT), there was no pattern of decreased bleeding with increased platelet count in the range of 6 × 109/L to 80 × 109/L in adult or pediatric patients.19-21 Even at counts <5 × 109/L, the increased uptick in bleeding rates was minimal. Bleeding was more common among allograft SCT recipients and in children, suggesting that clinical factors other than platelet count are important determinants of bleeding risk.22 A secondary analysis of The Trial of Prophylactic vs No-prophylactic Platelet Transfusions in Patients with Hematological Malignancies (TOPPS) explored risk factors for bleeding, including platelet count.23,24 The results indicated that a range of clinical factors are relevant to an increased number of days of bleeding, such as treatment plan (allogeneic hematopoietic SCT/chemotherapy), female sex, and pyrexia. The number of days with a platelet count <10 × 109/L was associated with developing a World Health Organization (WHO) grade 2 to 4 bleed, perhaps suggesting that the cumulative burden of thrombocytopenia, rather than isolated low platelet counts, alongside a history of recent bleeding events, would be a better guide for the decision for a prophylactic platelet transfusion. The lack of a clear relationship between severity of thrombocytopenia and risk of spontaneous bleeding also extends to other patient populations, with a poor correlation between the degree or severity of thrombocytopenia and the risk of bleeding or interventricular hemorrhage (IVH) in neonates (see later section “Case 2: pediatric patients and preterm neonates”).
Transfusion reactions
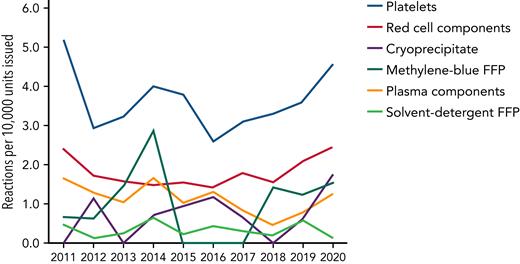
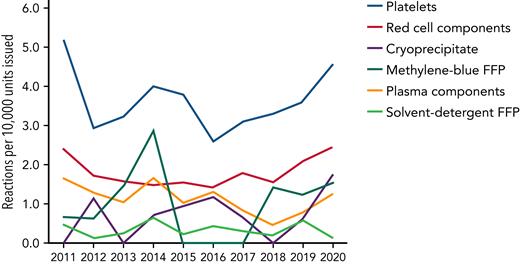
Platelets are the most commonly implicated component associated with transfusion reactions (Figure 1).25 Febrile nonhemolytic transfusion reactions and allergic reactions may occur at a reported frequency of 1 in 14 and 1 in 50 per transfusion unit, respectively.5 Although some of these reactions may be seen as minor by clinicians, recipients of platelet transfusion say that these episodes can be distressing, particularly when repeated. There is no evidence that prophylactic antipyretics or antihistamines reduce the incidence of transfusion reactions,26,27 but data suggest that fewer reactions occur in platelet products stored in platelet additive solutions, which contain less plasma.28 Sepsis from a bacterially contaminated platelet unit is the most frequent infectious complication from transfusing any blood product.29
Number of reactions reported per 10 000 components issued in the United Kingdom from 2011 through 2020. Although red blood cells are the most common blood component transfused, platelets account for the highest number of reactions. Convalescent plasma is not included. Reproduced with permission from SHOT.25 FFP, fresh frozen plasma.
Number of reactions reported per 10 000 components issued in the United Kingdom from 2011 through 2020. Although red blood cells are the most common blood component transfused, platelets account for the highest number of reactions. Convalescent plasma is not included. Reproduced with permission from SHOT.25 FFP, fresh frozen plasma.
Transfusion thresholds for platelets to prevent spontaneous bleeding
Although threshold-driven platelet transfusion forms the basis of current practice, it is essential to recall that early investigators were never able to observe a threshold effect.30 Available data (3 RCTs, 499 participants) suggest that lower (standard thresholds) of 10 × 109/L are not associated with more bleeding than are higher thresholds (20 × 109/L to 30 × 109/L).31 The threshold of 10 × 109/L is now commonly recommended by clinical guidelines (see Table 1).6 Yet, it is debatable whether some of these trials were adequately powered, particularly when bleeding is used as an outcome measure. A reanalysis of 1 trial suggested that differences may become apparent in a larger study.32 An important issue is what constitutes clinically important bleeding in patients with bone marrow failure or chemotherapy-induced thrombocytopenia. The WHO system for grading the severity of different bleeding events is often used, although it was developed as a system for reporting adverse events.33 Limitations include broad categories that may miss small changes in bleeding and definitions, such as bleeding requiring a transfusion, which, although pragmatic, may limit standardization, as thresholds for transfusion vary. Clinically significant bleeding in RCTs has often been applied as WHO grade ≥2, but such an outcome is a composite of different grades of bleeding.34 Overall, RCTs have reported highly different baseline rates of bleeding, raising further questions about the methodology and reporting of bleeding events.32,33 There are also likely to be differences between patients’ and clinicians’ perceptions of bleeding severity. The impact of bleeding on patients’ quality of life is unclear, as few studies have evaluated this aspect.35 Of course, the more severe types of bleeding events (eg, intracranial or intracerebral) are accepted as significant to patients and clinicians, but these events remain uncommon. Personal practices and review of severe bleeding events in RCTs reveals that major bleeding events often occur at platelet counts above the thresholds that would be considered indications for platelet transfusion. Finally, it is unclear whether less severe grades of bleeding predict more severe episodes. If there were data to support this progression of bleeding, it would have important implications for how we may use prophylactic platelet transfusions. Secondary analyses from the TOPPS RCT, found no evidence that minor bleeding predicted WHO grade 2 to 4 bleeding episodes,24 although an analysis of a different earlier data set showed different findings.36
Coming back to our case, what would happen if we omitted a platelet transfusion?
Does platelet transfusion prophylaxis have benefit?
The PLADO trial demonstrated that a high-dose platelet policy did not decrease rates of bleeding or the number of transfusion episodes per participant. Put another way, there was no evidence of a dose effect. A higher dose was associated, unsurprisingly, with an increase in the number of transfusion-related adverse events.19,31 This work is informing national discussions about minimum threshold specifications for platelet content, given ongoing concerns about supply and inventory management.37 Two later RCTs compared outcomes in patients allocated to a protocol of routine prophylaxis or no prophylaxis (only therapeutic).23,38 These trials were considered to challenge the dogma of the time, given that they supported a protocol of no-platelet transfusions irrespective of platelet count. Both trials reached recruitment targets, perhaps indicating that any risks that may have been found were more likely to be on the lower side. Noninferiority for rates of WHO grade 2 to 4 bleeding was close to being declared in the larger TOPPS trial. Moreover, in patients who underwent autologous transplantation (the largest subgroup), rates of WHO grade 2 to 4 bleeding were identical. The TOPPS trial was not powered to assess differences in severe bleeding at WHO grade 3 or 4, but this information was reported, as in all RCTs (6 cases in the no-prophylaxis group vs 1 case in the prophylaxis group; see later analysis).
Case management
Many patients who receive platelet transfusions, irrespective of transfusion policy, will continue to experience bleeding, and the impact of platelet transfusions on bleeding on subsequent days is unclear.20
Clinical factors other than platelet count are important determinants of bleeding.
Certain subgroups of patients (eg, autologous SCT) may not require prophylactic platelet transfusions, irrespective of platelet count.
A risk-adapted approach to platelet transfusions may be more prudent in our case, rather than applying a transfusion threshold platelet count of 10 × 109/L and this patient may not benefit from a prophylactic platelet transfusion.
Case 2: pediatric patients and preterm neonates
A preterm female neonate, born at 27 weeks gestational age, needed minimal respiratory support at postnatal day 4. Clinical examination revealed minimal oozing at the umbilical cord stump. The platelet count was 35 × 109/L.
IVH is a catastrophic complication in preterm neonates and is associated with a high likelihood of death or disability.39 To prevent this occurrence, neonatologists have traditionally adopted a more liberal approach toward platelet transfusion, with surveys suggesting that many clinicians apply prophylactic transfusion at platelet counts >50 × 109/L.40-42 Until recently, neonatologists had few data from RCTs but the Platelets for Neonatal Transfusion Study 2 (PLaNet-2/MATISSE) trial has now provided information on this question.43 Preterm neonates in the liberal threshold arm (<50 × 109/L) had a significantly higher rate of death or major bleeding within 28 days after randomization, when compared with restrictive transfusion (<25 × 109/L). A secondary analysis reported that the 25 × 109/L threshold was associated with absolute-risk reduction of different baseline risks across all groups. Another small trial compared a liberal (100 × 109/L) vs standard (20 × 109/L to 100 × 109/L) threshold for platelet transfusion in preterm infants with a hemodynamically significant patent ductus arteriosus.44 A liberal transfusion policy did not hasten closure of the patent ductus arteriosus but resulted in a higher incidence of IVH. In summary, available data favor a lower transfusion threshold for platelet transfusion in nonbleeding preterm neonates.45
Possible hypotheses to explain the mechanisms of harm in participants randomly allocated to liberal transfusion groups include the fluid shifts associated with transfusion volume and the proinflammatory effects of platelets, including inflammatory mediators that accumulate in platelets during storage and disruption of cerebral blood flow.39,45,46 An additional way to understand the risks of platelet transfusions would be to use hemovigilance systems. Unfortunately, most hemovigilance systems fail to clearly report pediatric and neonatal transfusions. Only 8% of reports submitted to SHOT (Serious Hazards of Transfusion) are pediatric cases, although these may be more common proportionately relative to adults.47 In the most recent 2020 report, there was an increase in reports related to febrile, allergic, and hypotensive reactions which appeared to be largely related to an unexplained increase in pediatric platelet transfusions.
Although there are no comparable RCT data in older children, international cohort studies in critically ill children indicate that most transfusions are given as prophylaxis to nonbleeding children, with significant variation in platelet thresholds and increments after transfusion.48 Many of these children, who will never develop bleeding complications, may be exposed unnecessarily to the risks of platelet transfusion.
Case management
RCT data support a lower platelet transfusion threshold (25 × 109/L) in nonbleeding preterm neonates.
Better hemovigilance reporting is needed for platelet transfusions in children.
In this case, with the absence of any clinically significant bleeding, it would be reasonable to withhold a platelet transfusion.
Case 3: critically ill adult
A 70-year-old man was admitted to the intensive care unit (ICU) with pneumococcal pneumonia. He was placed on a ventilator and treated with intravenous antibiotics. The platelet count was 17 × 109/L. The patient required insertion of a central venous catheter.
Thrombocytopenia is common in critically ill adults, and 5% to 20% will develop severe thrombocytopenia (<50 × 109/L) at some point in their ICU stay.49-51 The underlying etiology is multifactorial,52-56 but thrombocytopenia within the first 24 hours of ICU admission appears to be associated with increased 28-day mortality,57 along with a dysregulated host immune response.58 After patients with cancer, critically ill patients are the second largest group of platelet users.15 In a study of 29 ICUs in the United Kingdom, 9% of all patients received a platelet transfusion at some point during their ICU stay, many as prophylaxis. The range of platelet counts over which platelet transfusions are given to critically ill patients is wide,50,59,60 usually within 10 × 109/L to 50 × 109/L; the variation most likely reflects a lack of supporting evidence for best practice. Clinical guidelines have made inconsistent recommendations, often based on low-quality evidence (Table 1). Recent European Society of Intensive Care Medicine transfusion guidelines did not have enough evidence to make a recommendation regarding prophylactic platelet transfusion before an invasive procedure for platelet counts between 10 × 109/L and 50 × 109/L, and trials are urgently needed.61
Other guidelines5 have recommended transfusion thresholds of 10 × 109/L to 20 × 109/L, largely based on studies in patients with a hypoproliferative bone marrow as described in case 1. However, critically ill patients may also have acquired platelet dysfunction related to accompanying conditions (renal failure, trauma, and antiplatelet drugs), and bleeding may occur even with platelet counts >50 × 109/L.62 Given the routine use of ultrasound to guide insertion of central venous catheters, the incidence of major procedure-related bleeding is very low at ∼0.05% to 1%,63 which has implications for sample sizes for future studies.64 A substudy of a large RCT found that prophylactic platelet transfusions given to critically ill patients with thrombocytopenia were not associated with a reduction in the risk of major bleeding compared with that in patients without transfusion.65 One observational study found that preprocedural platelet transfusion in patients with thrombocytopenia (<100 × 109/L) scheduled to undergo interventional radiology procedures was not associated with a reduced risk of bleeding complications (defined as a requirement for a periprocedural red cell transfusion).66
The expected increase in platelet count from 1 platelet transfusion is between 12 × 109/L and 20 × 109/L,15,67 but this is highly variable in critically illness.48 Patients with underlying bone marrow failure may experience smaller increments in platelet count when compared with those without marrow failure.68 Although the absolute count may increase, it is unclear whether these transfused platelets function effectively, and there are few data on critically ill patients.69
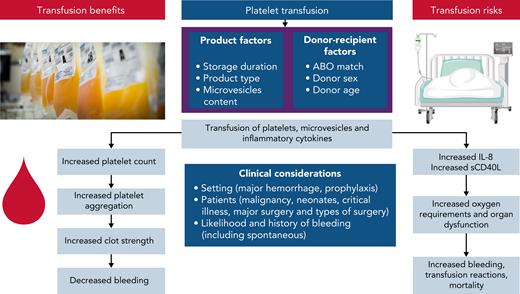
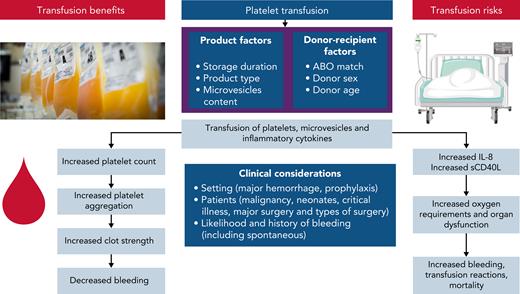
Platelet transfusions in critically ill patients are associated with risks, including acute respiratory distress syndrome, nosocomial infection, worsening organ failure, venous and arterial thrombosis, longer ICU stays, and increased mortality70-73 although there may be residual confounding by indication (sicker patients receive more platelet transfusions). The rates and mechanisms of harm need further investigation in critical illness but may result from proinflammatory mediators contained in platelet transfusions, including platelet-derived microvesicles and inflammatory cytokine release7,74-77 (Figure 2). Worsening oxygenation may also be associated with the mostly platelet-derived soluble form of CD40 ligand.73,78,79
A summary of putative mechanisms underlying the potential benefits and risks of platelet transfusions. Product and donation characteristics that may modify the efficacy and safety of platelet transfusions include ABO matching between donor and recipient, processing methods (eg, pathogen reduction technology, and storage media), and storage duration. Platelet and leukocyte activation leads to accumulation of proinflammatory cytokines (IL-1, -6, and -8 and transforming growth factor-β), soluble CD40 ligand, and formation of microvesicles. Platelet microvesicles become more numerous and injurious during storage and may trigger a recipient reaction, mediated by their molecular cargo, resulting in further inflammatory cytokine release. Platelet microparticles may downregulate macrophages and impair the reactivity of dendritic cells.
A summary of putative mechanisms underlying the potential benefits and risks of platelet transfusions. Product and donation characteristics that may modify the efficacy and safety of platelet transfusions include ABO matching between donor and recipient, processing methods (eg, pathogen reduction technology, and storage media), and storage duration. Platelet and leukocyte activation leads to accumulation of proinflammatory cytokines (IL-1, -6, and -8 and transforming growth factor-β), soluble CD40 ligand, and formation of microvesicles. Platelet microvesicles become more numerous and injurious during storage and may trigger a recipient reaction, mediated by their molecular cargo, resulting in further inflammatory cytokine release. Platelet microparticles may downregulate macrophages and impair the reactivity of dendritic cells.
The effects of platelet transfusion on inflammation and hemostasis in critically ill patients may be further modified by donation characteristics of the platelet unit, but this has been poorly studied in critical illness. In the setting of prophylaxis in hematological cancers, a secondary analysis of the PLADO trial showed that platelet source, ABO compatibility, and duration of storage did not affect bleeding rates, although platelet increments were generally higher with transfusions of apheresis platelets, ABO-identical platelets, and platelets stored 3 days vs 4 to 5 days.80 In other studies, ABO-incompatible transfusions have been associated with poor platelet recovery and increased mortality.80-82 Data support possible associations between donation characteristics and outcomes and this reiterates the importance for further study of how donation characteristics impact on outcomes including in settings such as critical illness.83-86
Are there alternatives or better tests than a platelet count to predict bleeding?
Viscoelastic hemostatic assays (VHAs) such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are increasingly being used to guide transfusion therapy in critical care, liver disease, cardiac surgery, and obstetrics.87 Such tests provide a global assessment of coagulation at the bedside to deliver targeted transfusion of blood products. Limited evidence suggests that VHA-guided therapy may reduce transfusion requirements in patients who undergo cardiac or liver surgery or have obstetric hemorrhage.87 In a small number of patients with thrombocytopenia and hematological malignancy, ROTEM-measured clot firmness and TEG-measured α angle have been shown to correlate better with bleeding than platelet count.88,89 A systematic review of the use of TEG/ROTEM in patients with sepsis found that these tests may be useful for diagnosing alterations in coagulation in sepsis, such as impaired fibrinolysis, when compared with standard laboratory tests.90 Larger studies are needed to answer whether correcting abnormal TEG/ROTEM values is associated with improvements in clinical outcomes in critically ill patients, including use of platelets.
Case management
Platelet transfusions may be associated with increased morbidity and mortality in critically ill patients, with limited evidence of benefit, and RCTs are needed.
The role of TEG/ROTEM in stable, nonbleeding, critically ill patients requires further investigation.
It would be reasonable to withhold a platelet transfusion in this case. The procedure should be performed by an experienced operator, using ultrasound guidance, to minimize the risk of bleeding.
Cases 4 and 5: traumatic hemorrhage and intracranial bleeding
In case 4, a 25-year-old man was admitted to the emergency department after a serious traffic accident. The massive hemorrhage protocol (MHP) was activated. Despite an initial normal platelet count of 140 × 109/L and an intraoperative transfusion of 1 dose of platelets, the repeated platelet count was 49 × 109/L.
Trauma-induced coagulopathy is an overall failure of the coagulation system mediated by protein C activation, hyperfibrinolysis secondary to release of tissue plasminogen activator, and rapid depletion of fibrinogen.91-93 Platelet dysfunction is common after major trauma and may be associated with increased mortality, even when the platelet count is within the normal reference range.94 Significant thrombocytopenia is considered a late event in major hemorrhage. The cornerstones of management include timely and balanced administration of blood components with control of bleeding (either surgical or radiological).91 As a pragmatic approach, guidelines often recommend that platelet transfusions be given to maintain the platelet count at >50 × 109/L in trauma bleeding.95,96 MHPs may have improved outcomes in many observational studies, including mortality, but these studies did have significant survivorship or immortal time bias (ie, participants must be alive long enough to receive the intervention).91,97,98
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial randomly allocated 680 patients with traumatic bleeding to receive either high or low ratios of plasma and platelets to red blood cells (1:1:1 vs 1:1:2). There was no difference in all-cause mortality between the groups, but in secondary analyses, patients in the high-plasma and platelet ratio group (1:1:1) had a reduced risk of dying from exsanguination in the first 24 hours, although not a prespecified outcome.99 A post hoc analysis suggested that early platelet administration was associated with improved hemostasis and reduced mortality.100
TEG/ROTEM-directed transfusion algorithms have been used to guide a more individualized approach for blood components and platelets in major traumatic bleeding. These have been widely reported, and the most common abnormality observed is a reduction in clot strength.101 Two small, single-center RCTs have reported reductions in mortality and clinically relevant bleeding using TEG102 and ROTEM.103 However, the recent Implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy (ITACTIC)104 multicenter RCT compared standard MHPs, using conventional coagulation tests vs VHA guided algorithms, but found no difference in the number of patients who were alive and free of massive transfusion at 24 hours.104
Case management
Trauma-induced coagulopathy can lead to platelet loss and consumption.
The impact of TEG/ROTEM on clinical outcomes is unclear.
In our case, priority should be given to identifying and controlling the source of bleeding, either radiologically or surgically. The platelet count should (pragmatically) be maintained at 50 × 109/L with platelet transfusions.
In case 5, a 78-year-old woman was admitted to the emergency department with increasing confusion. She was taking clopidogrel. A computed tomography scan revealed an acute subdural hematoma but neurosurgery is not currently planned. Her platelet count was 271 × 109/L.
Approximately 2 million nontraumatic (spontaneous) intracerebral hemorrhages (ICHs) occur worldwide each year,105 and in high-income countries more than a quarter of patients who experience an ICH will be taking antiplatelet therapy.106 Platelet transfusions have been commonly used (and continue to be) in those patients to reduce ICH volume. However, the results of the Platelet Transfusion in Cerebral Hemorrhage (PATCH) RCT have challenged this indication.107 Patients with a supratentorial ICH, use of antiplatelet medication for at least 7 days prior, and Glasgow Coma Scale >8 were randomly allocated to receive standard care or standard care with platelet transfusion within 90 minutes of diagnostic brain imaging. Platelet transfusion was associated with an increased the risk of death or dependence in patients receiving antiplatelet therapy and presenting with an acute ICH. It is unclear whether the findings are generalizable to an increasing number of patients who are now taking agents such as clopidogrel, and various methodological limitations have been described,108 but this trial again shows the potential harm of platelet transfusions.
Case management
Despite its limitations, PATCH is the best available RCT evidence on this topic, and a platelet transfusion is not indicated.
Further studies are needed on patients prescribed antiplatelet medication and the underlying mechanisms between platelet transfusions and clinical outcomes.
Discussion
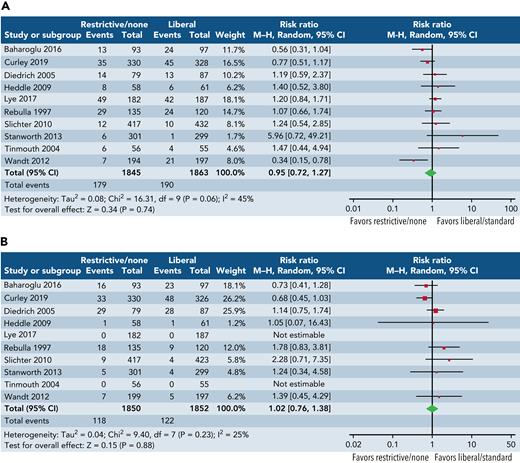
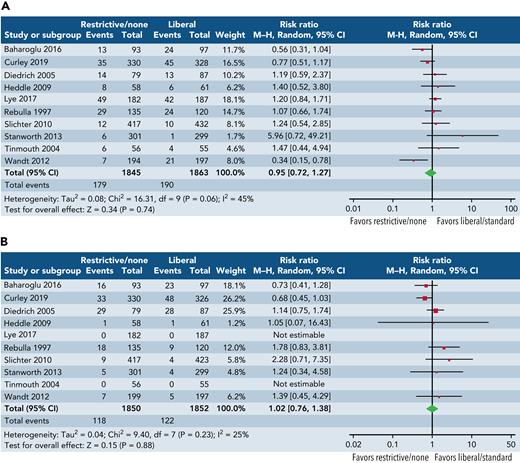
This review has highlighted the uncertainty about the perceived benefits of platelet transfusions alongside risks. To further illustrate this uncertainty, we undertook an exploratory meta-analysis of major bleeding (grade ≥3) and mortality (Figure 3) across all randomized trials, recruiting 100 patients or more, irrespective of clinical setting. The results of this analysis are hypothesis generating, but the pooled estimates of effect suggest no consistent impact of platelet transfusions in reducing bleeding or mortality, although the confidence intervals are wide and may encompass potentially important clinical differences. Platelets clearly have many biological roles beyond hemostasis, and we need a better understanding of the clinical consequences of these immune effects.109,110 Given the uncertainties regarding the role of platelet transfusions, there is interest in alternatives to platelet transfusion111,112 that may include drugs and factors that stimulate endogenous production of platelets or von Willebrand factor (eg, thrombopoietin, desmopressin),113 target fibrinolysis (eg, tranexamic acid),114 or increase fibrin and fibrinogen (eg, fibrinogen concentration, recombinant factor VIIa) or use of artificial platelets or platelet membranes (eg, nanoparticles).115 Of note, a recent trial reported no evidence of an effect of tranexamic acid in reducing WHO grade 2+ bleeding in adult patients with thrombocytopenia who undergo therapy for hematological malignancy116; results of an ongoing trial are awaited.117
Exploratory forest plots of the effect of 2 strategies. Restrictive or no prophylaxis strategy (as defined by the study authors) vs a liberal strategy (as defined by the study authors) on major bleeding (A) and all-cause mortality (B) from randomized trials of platelet transfusions recruiting ∼100 patients or >100 patients. Study definitions vary and analysis included all settings although more commonly hematological cancers. No prophylaxis strategies for platelet transfusion were applied unless there was evidence of clinically significant bleeding. Restrictive transfusion strategies advocated platelet transfusions at thresholds ranging from 10 × 109/L to 25 × 109/L. Slichter et al19 compared 3 different doses of platelets; for the purposes of this analysis, we selected the low- and high-dose arms.
Exploratory forest plots of the effect of 2 strategies. Restrictive or no prophylaxis strategy (as defined by the study authors) vs a liberal strategy (as defined by the study authors) on major bleeding (A) and all-cause mortality (B) from randomized trials of platelet transfusions recruiting ∼100 patients or >100 patients. Study definitions vary and analysis included all settings although more commonly hematological cancers. No prophylaxis strategies for platelet transfusion were applied unless there was evidence of clinically significant bleeding. Restrictive transfusion strategies advocated platelet transfusions at thresholds ranging from 10 × 109/L to 25 × 109/L. Slichter et al19 compared 3 different doses of platelets; for the purposes of this analysis, we selected the low- and high-dose arms.
There are gaps in research and development for the platelet product, including the role of cryopreserved and cold-storage platelets, particularly in military settings or remote hospitals where the ability to provide standard platelets is challenging because of their short shelf life. Recent research has highlighted the uncertain clinical impact of the transfusion of platelets with qualitative defects in donations.118 Cryopreserved platelets have been approved for general civilian use and for military use in some countries.91 Small pilot RCTs of cryopreserved119 or cold-stored platelets120 in patients who undergo major cardiac surgery have shown no signs of harm and may have added hemostatic benefits. A large confirmatory trial of cold-stored platelets is ongoing (registered on https://clinicaltrials.gov as #NCT04834414).
Work on larger data sets, using advanced statistical techniques, would enable us to identify the patient at lower or greater risk of bleeding, allowing us to target interventions like platelet transfusions. Sufficient platelets would be administered to optimize vascular integrity and improve hemostasis and improve patient outcomes, while avoiding unnecessary routine transfusions of platelets with associated risks and costs.121,122 A high platelet count unit containing platelets that are more primed for activation and aggregation (hyperreactive) may be preferentially allocated for major bleeding. In contrast, a low platelet count unit may be indicated in the setting of nonbleeding prophylaxis, where these platelets support and maintain endothelial function, without adding to prothrombotic and proinflammatory risks.123
We must design more cost-efficient adaptive trials, rather than those based on comparisons between only 2 arguably arbitrarily defined thresholds, which may not identify the actual “sweet spot” or optimal threshold where platelet transfusions have maximal benefit in a specific patient subgroup. The future offers opportunities for a precision-medicine–based approach for platelet transfusions with optimized donor-recipient matching.
Acknowledgments
The authors thank Darrell Triulzi, Rebecca Cardigan, and Peter Watkinson for constructive feedback and discussions that refined the manuscript.
Authorship
Contribution: Both authors designed, reviewed, and approved the final submission.
Conflict-of-interest disclosure: S.J.S. reports receiving funds from government sources (National Institutes of Health Research and NHS Blood and Transplant) for research in the field of platelet transfusion. S.J.S. and A.S. are co-investigators on the NIHR-funded Threshold for Platelet (T4P) trial (NIHR 131822).
Correspondence: Simon J. Stanworth, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom and NHS Blood and Transplant, Level 2, John Radcliffe Hospital, Headley Way, Headington, Oxford OX3 9BQ, United Kingdom; e-mail: simon.stanworth@nhsbt.nhs.uk.