In this issue of Blood, Sperling et al1 evaluate the association between chemo- and radiation therapies (CRT) and therapy-related myeloid neoplasms (t-MNs) and demonstrate that exposure to lenalidomide is associated with the development of t-MNs harboring TP53 mutations.
This effect appears to be specific to lenalidomide as other thalidomide analogs such as pomalidomide and iberdomide do not confer a selective advantage to TP53-mutant clones in vitro. In addition, lenalidomide specifically selects for TP53-mutant clones and not for other mutations associated with clonal hematopoiesis (CH). Overall, these findings identify a specific therapy associated with the development of TP53-mutant t-MN and suggest that alteration of lenalidomide-based therapies may offer a strategy to mitigate the risk of patients developing t-MNs.
t-MNs arise as a late effect of CRT administered for another condition, typically solid and nonmyeloid cancers or autoimmune disease. The latency period between diagnosis of the primary disease and occurrence of t-MN ranges between months to years and is influenced by the type of preceding CRT, the cumulative dose of therapy, and dose intensity.2 Recent studies indicate that CRT induces clonal selection of preexisting mutant hematopoietic stem and progenitor cells (HSPCs) that do not undergo apoptosis in response to cytotoxic or DNA damaging therapies.2 Such therapies have been associated with the outgrowth of mutant clones harboring mutations in TP53 as well as PPM1D, which may be detectable years before CRT exposure.3 Avoiding the development of t-MN is critically important because t-MN patients experience inferior outcomes compared with those with de novo myeloid neoplasms.4 It is therefore critical to understand the mechanistic basis of clonal selection underlying the pathogenesis of t-MN to determine possible modifications to the therapeutic regimen and minimize the risk of t-MN development.
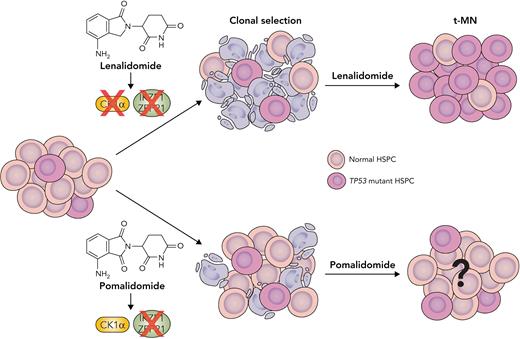
In this study, the investigators analyzed the clinical features of 416 patients diagnosed with t-MN based on the 2016 World Health Organization classification scheme. Mutational profiles showed that ≥1 mutation was detected in 85% of patients, with a predominance of TP53 (37%) and PPM1D (19%) mutations. Compared with a cohort of acute myeloid leukemia(AML)/myelodysplastic syndrome(MDS) patients without prior CRT, the investigators found that TP53 and PPM1D mutations were significantly more frequent in t-MNs. When the type of prior CRT was taken into account, correlations between complex karyotype and platinum agents as well as chromosome 7 abnormalities and alkylating and platinum agents were identified, as expected. In addition, a significant association was identified between TP53 mutations and prior lenalidomide therapy; the duration of exposure also correlated with the risk of t-MN. To determine the potential mechanisms of clonal selection, the authors showed that, similar to their prior studies,5,6 lenalidomide induces CK1α degradation, leading to p53-dependent apoptosis in wild-type (WT), but not TP53-mutant, HSPCs. In addition, loss of p53 in Csnk1a1−/+ mouse HSPCs conferred resistance to lenalidomide treatment (see figure). Moreover, the authors showed that the competitive advantage of TP53-mutant HSPCs is specific to lenalidomide because pomalidomide did not induce CK1α degradation or p53-dependent cell death. Finally, using a multiplexed CRISPR-Cas9 system, the investigators demonstrated that lenalidomide confers a selective advantage to TP53-mutant HSPCs, but not HSPCs carrying other common CH-associated mutations (DNMT3A, TET2, ASXL1, EZH2, and PPM1D).
Thalidomide analogs exhibit differential effects in driving selection of TP53-mutant HSPC clones. Thalidomide and its analogs function by increasing the affinity of the CRL4CRBN E3 ubiquitin ligase for specific protein substrates, including key transcription factors IKZF1, ZFP91, and casein kinase 1α (CK1α), which are critical for HSPC survival. Lenalidomide leads to a specific positive selection of TP53-mutant clones by inducing CK1α degradation and p53-dependent apoptosis in normal HSPCs. Another thalidomide analog, pomalidomide, induces degradation of IKZF1 and ZFP91 but does not induce CK1α degradation or apoptosis, and thus HSPCs do not experience the same selection pressure. Subsequent outgrowth of TP53-mutant HSPC clones in the context of lenalidomide treatment increases the risk of t-MN development. However, it is unknown if pomalidomide therapy is a risk factor for t-MN. Professional illustration by Somersault18:24.
Thalidomide analogs exhibit differential effects in driving selection of TP53-mutant HSPC clones. Thalidomide and its analogs function by increasing the affinity of the CRL4CRBN E3 ubiquitin ligase for specific protein substrates, including key transcription factors IKZF1, ZFP91, and casein kinase 1α (CK1α), which are critical for HSPC survival. Lenalidomide leads to a specific positive selection of TP53-mutant clones by inducing CK1α degradation and p53-dependent apoptosis in normal HSPCs. Another thalidomide analog, pomalidomide, induces degradation of IKZF1 and ZFP91 but does not induce CK1α degradation or apoptosis, and thus HSPCs do not experience the same selection pressure. Subsequent outgrowth of TP53-mutant HSPC clones in the context of lenalidomide treatment increases the risk of t-MN development. However, it is unknown if pomalidomide therapy is a risk factor for t-MN. Professional illustration by Somersault18:24.
t-MNs associated with prior lenalidomide therapy almost exclusively arise in patients with a primary diagnosis of multiple myeloma (MM), as standard induction and maintenance therapy for MM patients may include a combination of proteasome inhibitor (bortezomib), thalidomide analog (lenalidomide), and antiinflammatory agent (dexamethasone).7,8 Thus, one important question that arises is whether lenalidomide drives clonal outgrowth of TP53-mutant HSPCs alone or if proteasome inhibitors provide an additional advantage to mutant clones. To address this issue, the investigators performed a number of analyses to support their conclusion that the risk of t-MN in MM patients is driven by lenalidomide and not proteasome inhibitor therapy. First, the authors performed a multicollinearity test for multivariate analysis and found that thalidomide analogs exhibited the highest variance inflation factor as a measure of the severity of multicollinearity. Second, in vitro studies using WT and TP53-mutant mouse HSPCs using the Hoxb8 system demonstrated the inherent resistance of TP53-mutant clones to proteasome inhibitors such as bortezomib and carfilzomib is among the lowest of all drugs tested. Third, the investigators showed that localized exposures like radiation therapy enrich different mutational profiles, more similar to the mutational spectrum observed in de novo disease. It is important to note that prior large-scale epidemiological studies and data from clinical trials also have demonstrated an association between lenalidomide exposure and t-MN, although the mechanistic basis of this association was unknown.9,10 Collectively, these findings indicate that proteasome inhibitor therapy likely does not significantly contribute t-MN risk in MM patients.
While this study represents an important advance in the field of t-MNs and suggests modification of treatment regimens containing lenalidomide, some outstanding questions remain. First, while lenalidomide appears to promote the outgrowth of TP53-mutant clones, it is not clear whether other thalidomides such as pomalidomide (currently administered to relapsed MM patients who have already been exposed to lenalidomide) may drive clonal selection populations harboring other CH mutations. In addition, it is currently unclear what strategy should be used to minimize the risk of t-MN in MM patients—would this require the elimination of lenalidomide from MM therapy, switching to another thalidomide during the maintenance phase to shorten the exposure time of patients to lenalidomide-based therapy, or would highly sensitive screening of MM patients for TP53-mutant CH before therapy be sufficient to appropriately select lenalidomide therapy, or should the same approach be used to monitor patients during maintenance therapy? Current trials and future prospective studies investigating the efficacy of newer thalidomide analogs that do not depend on TP53-dependent mechanisms and assessing TP53-mutant CH status during lenalidomide maintenance therapy promise to address some of these questions. Overall, the current study reinforces the notions that clonal selection and outgrowth of premalignant HSPC clones are significantly modulated by the interplay between genotype and extrinsic stressors such as choice of therapy and that the genetic origins of t-MNs are distinct from de novo AML/MDS.
Conflict-of-interest disclosure: The authors declare no competing financial interests.