Key Points
The ORR and CR rates at completion of PET-adapted Nivo/Nivo+ NICE were 93% and 91%.
Two-year PFS in all patients was 72%, and 94% in the 33 patients who bridged directly to AHCT after protocol therapy (26 after Nivo only).
Abstract
This phase 2 trial evaluated PET-adapted nivolumab alone or in combination with ifosfamide, carboplatin, and etoposide (NICE) as first salvage therapy and bridge to autologous hematopoietic cell transplantation (AHCT) in relapsed/refractory (RR) classical Hodgkin lymphoma (cHL). Patients with RR cHL received 240 mg nivolumab every 2 weeks for up to 6 cycles (C). Patients in complete response (CR) after C6 proceeded to AHCT, whereas patients with progressive disease at any point or not in CR after C6 received NICE for 2 cycles. The primary endpoint was CR rate per the 2014 Lugano classification at completion of protocol therapy. Forty-three patients were evaluable for toxicity; 42 were evaluable for response. Thirty-four patients received nivolumab alone, and 9 patients received nivolumab+NICE. No unexpected toxicities were observed after nivolumab or NICE. After nivolumab, the overall response rate (ORR) was 81%, and the CR rate was 71%. Among 9 patients who received NICE, all responded, with 8 (89%) achieving CR. At the end of protocol therapy, the ORR and CR rates were 93% and 91%. Thirty-three patients were bridged directly to AHCT, including 26 after Nivo alone. The 2-year progression-free survival (PFS) and overall survival in all treated patients (n = 43) were 72% and 95%, respectively. Among 33 patients who bridged directly to AHCT, the 2-year PFS was 94% (95% CI: 78-98). PET-adapted sequential salvage therapy with nivolumab/nivolumab+NICE was well tolerated and effective, resulting in a high CR rate and bridging most patients to AHCT without chemotherapy. This trial was registered at www.clinicaltrials.gov #NCT03016871.
Introduction
About 10% to 30% of patients with classical Hodgkin lymphoma (cHL) will have primary refractory or relapsed (RR) disease.1-5 Standard therapy for RR cHL is salvage combination chemotherapy followed by autologous hematopoietic cell transplantation (AHCT) in chemosensitive patients.6-10 Achievement of a positron emission tomography (PET)-negative complete response (CR) prior to AHCT is one of the most important predictors of outcomes after AHCT.11-14 Standard salvage chemotherapy is associated with PET− CR in 54% to 73% of patients.15-20 Incorporation of the anti-CD30 antibody drug conjugate, brentuximab vedotin (BV), into salvage therapy has been studied either in sequence or in combination with chemotherapy, yielding CR rates ranging from 68% to 83%.21-29 In sequential studies, BV monotherapy as initial salvage can result in CR for 27% to 43% of patients prior to AHCT, without the use of chemotherapy.21,23
Nivolumab (Nivo) is an anti-PD-1 antibody that restores effective antitumor immune responses and is tolerable and effective in patients with RR cHL.30-33 BV+Nivo as first salvage therapy was a well-tolerated regimen free of traditional chemotherapy that yielded a CR rate of 67% and 3-year progression-free survival (PFS) rate of 77% as a bridge to AHCT in patients with RR cHL.34,35 However, with increasing use of frontline BV following its approval for advanced-stage cHL, BV-based salvage therapy may not be optimal for many patients with RR cHL. We conducted a multicenter phase 2 trial evaluating PET-adapted sequential Nivo followed by Nivo combined with ifosfamide, carboplatin, and etoposide (NICE) as first salvage therapy in RR cHL to evaluate the role of Nivo as salvage therapy independent of BV as well as the safety and efficacy of NICE in patients who did not achieve CR with Nivo alone.
Methods
Patients
This was a multicenter phase 2 trial in patients with initial RR cHL evaluating response-adapted salvage therapy using sequential Nivo/NICE. Eligible patients were 18 years or older weighing >40 kg. Patients were required to have histologically confirmed CD30+ cHL that was either primary refractory or had relapsed after initial therapy. Primary refractory cHL was defined as less than CR at completion of initial therapy. Additional criteria for inclusion were as follows: Eastern Cooperative Oncology Group performance status 0 to 2, diffusing capacity of the lungs for carbon monoxide ≥60% of predicted, total bilirubin ≤1.5× upper limit of normal (ULN), aspartate aminotransferase/alanine aminotransferase (AST/ALT) ≤3.0× the institutional ULN, and activated thromboplastin time/international normalized ratio <1.5× ULN and partial thromboplastin time (activated partial thromboplastin time) <1.5× ULN. For patients with cHL involvement of the liver, total bilirubin ≤5.0× ULN, and AST/ALT ≤ 5.0× ULN were allowed. Hematologic parameters required were absolute neutrophil count ≥1500/μL, platelet count ≥75 000/μL, and hemoglobin ≥8.5 g/dL; growth factor support and transfusion were allowed prior to the start of study treatment to achieve the target levels. Patients with active hepatitis B or C infection were ineligible. Patients with HIV were eligible if HIV viral load was undetectable or unquantifiable and CD4 count >300 on highly active antiretroviral therapy. Prior exposure to PD-1 or PD-L1 blockade was not allowed. Patients with recent myocardial infarction, congestive heart failure, uncontrolled angina, or acute uncontrolled arrhythmia were excluded. All patients provided informed consent for participation in the clinical trial. The study was approved by the Institutional Review Board and conducted in accordance with the principles of the Declaration of Helsinki.
Study treatment
Patients received 240 mg Nivo administered IV over ∼30 minutes every 2 weeks for 3 cycles (14 days per cycle). PET–computed tomography (CT) was performed to assess disease response after the first 3 cycles of Nivo, and the results determined the subsequent treatment (Figure 1). Patients in CR or partial response (PR) received another 3 cycles of Nivo for a total of 6 cycles followed by another PET-CT scan. Patients in CR after 6 cycles of Nivo proceeded to AHCT. Patients who had progressive disease (PD) after 3 cycles or 6 cycles of Nivo proceeded to receive 2 cycles (21 days per cycle) of NICE: Nivo 240 mg day 1, etoposide 100 mg/m2 IV on days 1 to 3, carboplatin area under the curve 5 (750 mg maximum) IV on day 2, ifosfamide IV 5000 mg/m2 on day 2. Patients with stable disease (SD) after 3 cycles of Nivo could proceed with an additional 3 cycles of Nivo or proceed to NICE at the discretion of the treating investigator. Patients with SD or PR after 6 cycles of Nivo received NICE. Following NICE, patients in PR or CR could proceed to AHCT, whereas patients with SD or PD were removed from the study. Routine antiemetic prophylaxis was administered with each cycle of ICE per institutional standards and included 12 mg oral or IV dexamethasone on day 1, and 8 mg on days 2 and 3. Patients received granulocyte colony-stimulating factor support and antimicrobial prophylaxis per institution guidelines following each cycle of NICE. Stem cell mobilization, AHCT, and post-AHCT consolidation were performed at the discretion of the treating investigator according to institutional practices after the completion of study therapy.
Study assessments and endpoints
Safety was monitored continuously, with toxicities assessed using the Common Terminology Criteria for Adverse Events v4.03. Engraftment syndrome (ES) was defined according to established criteria.36 Unacceptable toxicity was defined as any hematologic or nonhematologic grade 3/4 toxicity that did not resolve to a grade 1/2 within 14 days and considered at least possibly related to Nivo and/or NICE, or any other regimen-related cause of death. PET-CT scans were performed after 3 cycles of Nivo, 6 cycles of Nivo (if applicable), and again after 2 cycles of NICE (when applicable). Response assessment was performed by investigators according to the 2014 Lugano classification.37 The primary endpoints were CR rate at the end of protocol therapy, and toxicity in the safety monitoring segment of the study. Secondary endpoints included estimates of overall response rate (ORR; [CR+PR]), response duration, overall survival (OS) and PFS, CD34+ yield, and proportion of patients who collected ≥2 × 106 CD34+ cells per kg. For patients who underwent AHCT: time to neutrophil and platelet engraftment, nonrelapse mortality, and relapse-progression incidence were also estimated.
Analysis of quantitative PET parameters
Total metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were assessed to evaluate the association between end of treatment response. Detailed methods for image acquisition and MTV/TLG protocol are described in the supplemental Methods, available on the Blood Web site.
mIF tumor analysis
When available, we analyzed formalin-fixed paraffin-embedded tumor samples obtained at relapse prior to treatment and at diagnosis using quantitative, automated, 7-color multispectral immunofluorescence (mIF) staining with multispectral imaging to examine the density of and spatial relationships between immune cells, immune checkpoint proteins, and tumor cells in the cHL tumor microenvironment (TME). Two mIF panels for cHL were used: (1) CD3, CD4, CD8, PD-1, CD30, DAPI; (2) CD56, CD163, CD79a, PAX5, PD-L1, CD30, DAPI. mIF images were examined using QuPath v0.3.0 bioimage analysis software to measure density of cell types and perform nearest neighbor analysis (supplemental Methods).
Trial design and statistical considerations
Trial design characteristics
This phase 2 trial implemented a Simon Two-Stage Optimal Design38 with the overall sample size determination based on the desire to discriminate a promising CR rate of 70% from a disappointing CR rate of 50% using a type I error rate of 0.05 and power of 80%. At stage one, 15 subjects were entered on the study. If ≤8 CRs were seen, the study would be terminated. If at least 9 subjects achieved a CR, the trial would continue to the second stage. At stage two, 28 additional subjects were entered. At the end of stage 2, if 27 or more subjects experienced a CR, the combination would be considered worthy of further study.
Safety monitoring segment
The trial included safety monitoring for the first 6 patients treated with the NICE during the first cycle. If ≤1/6 patients experienced unacceptable toxicity, then the study would be allowed to continue.
Analysis plan
Baseline characteristics and toxicities were summarized using descriptive statistics. Response rates were calculated as the percent of evaluable patients that had objective response by radiographic imaging, and 95% Clopper-Pearson confidence limits were calculated. Quantitative PET parameters (MTV and TLG) were compared between 2 response groups using Wilcoxon rank sum test. Survival estimates were calculated based on the Kaplan-Meier product-limit method, and 95% confidence intervals (CIs) were calculated using the logit transformation and the Greenwood variance estimate. All calculations were performed using SAS version 9.4 (SAS Institute, Cary, NC) or R v3.6.3. Trial data were locked for analysis on November 24, 2021.
Results
Patients
Forty-three patients were enrolled and received study therapy. Baseline characteristics are listed in Table 1. Twenty-six patients were male (60%). Median age was 35 years (range, 18-70); 4 (9%) patients received BV as part of frontline therapy, and 19 (44%) patients had primary refractory cHL. At baseline, 26 (60%) patients had advanced stage, 35% had B symptoms, and 19% had bulky disease (>5 cm).
Treatment disposition and safety
All 43 patients were evaluable for safety, and 42 patients had a response assessment and were evaluable for efficacy. Thirty-nine patients completed Nivo monotherapy, and 4 patients discontinued prior to completing Nivo monotherapy. Three patients discontinued because of toxicity during Nivo monotherapy (1 patient with grade 4 encephalitis, 1 patient with grade 2 pneumonitis, 1 patient with grade 2 thyroiditis in CR after 2 cycles and proceeded to AHCT), and 1 patient died of sepsis owing to an untreated dental abscess during Nivo monotherapy that was unrelated to study treatment. One patient withdrew consent after completing Nivo monotherapy (refused NICE). Nine patients went on to receive NICE. In total, 38 (88%) patients completed all intended protocol therapy.
The most common adverse events (AEs) attributed to Nivo monotherapy (n = 43, all grades) were fatigue (33%), maculopapular rash (19%), arthralgia (16%), fever (16%), nausea (16%), and leukopenia (16%) (Table 2). Three patients had grade ≥3 Nivo-related AEs, including 1 patient with grade 4 altered mental status requiring intubation (owing to immune-related encephalitis) and grade 3 tumor lysis syndrome, 1 patient with grade 3 thrombocytopenia, and another patient with grade 4 lymphopenia. Among 9 patients who received NICE, no unacceptable toxicities were noted. The most common NICE-related AEs (n = 9, all grades) were nausea (78%), anemia (67%), ALT elevation (56%), fatigue (56%), and vomiting (56%) (Table 3). The most common grade ≥3 NICE-related AEs were 2 patients each with neutropenia and hypophosphatemia, and 1 patient with febrile neutropenia, colitis, syncope, and leukopenia (supplemental Table 1). Possible immune-related AEs included maculopapular rash in 8 patients (Nivo, all grade 1 to 2), thyroiditis in 3 patients (Nivo, all grade 1 to 2), 2 patients with grade 1 acneiform rash, and 1 patient each with grade 4 encephalitis (Nivo), grade 3 colitis (NICE), grade 2 adrenal insufficiency (Nivo), grade 2 pneumonitis (Nivo), grade 2 pustular rash (Nivo), and grade 1 bullous pemphigoid (Nivo).
Treatment response
Among the 42 evaluable patients, the ORR and CR rate after 3 cycles of Nivo were 88% (37/42) and 62% (26/42), respectively (includes n = 1 patient assessed after 2 cycles; Figure 1). Thirty-seven patients proceeded to receive 3 additional cycles of Nivo, and the ORR and CR after 6 cycles in these 37 patients were 89% (33/37) and 78% (29/37), respectively. Four patients had objective response after 3 cycles of Nivo but experienced PD after completing 6 cycles of Nivo. The end of Nivo ORR and CR rate among all 42 evaluable patients were 81% (34/42) and 71% (30/42), respectively. One patient with PD after Nivo refused further treatment with NICE. Among the 9 patients who received NICE, all 9 (100%) patients responded, with 8 (89%) patients achieving CR. At the end of all protocol therapy (Nivo or Nivo/NICE), the ORR and CR rates were 93% (39/42) and 91% (38/42), respectively, among evaluable patients, and 91% and 88% among all-treated patients (Figure 1), respectively. Response rates were similar in patients with primary refractory and relapsed cHL (Table 4). Among the 4 patients who received BV as part of their frontline therapy, 3 achieved CR with Nivo and 1 had PD after 3 cycles of Nivo and then PR after NICE.
Survival outcomes
The median follow-up time among all patients was 30.7 months (range, 2.4-45.8). The 2-year PFS and OS in all treated patients (n = 43) were 72% (95% CI: 56-83) and 95% (95% CI: 82-99), respectively (Figure 2). Patients who were primary refractory (n = 19) had a 2-year PFS of 79% (95% CI: 53-92) as compared with a 2-year PFS of 67% (95% CI: 44-82) in patients who were relapsed (n = 24).
Survival outcomes. (A) PFS in all treated patients, (B) PFS in patients who proceeded directly to AHCT after protocol therapy, and (C) OS in all treated patients.
Survival outcomes. (A) PFS in all treated patients, (B) PFS in patients who proceeded directly to AHCT after protocol therapy, and (C) OS in all treated patients.
Transplant outcomes
Thirty-three (77%) patients proceeded to AHCT directly after protocol therapy, including 26 (60%) patients who underwent AHCT immediately after Nivo monotherapy. Among 32 patients with stem cell mobilization and collection data available, the median number of apheresis sessions required was 2 (range, 1-4). The median total number of CD34+ cells per kg harvested were 4.8 × 106 (range, 3.1-16.2). The stem cell mobilization regimens used are listed in supplemental Table 2. The median number of days to neutrophil and platelet engraftment were 11 (range, 9-14) and 16 (range, 8-29), respectively. All patients who underwent AHCT directly after protocol therapy received carmustine, etoposide, cytarabine, and melphalan conditioning (BEAM) except for 1 patient who received CD25-directed radioimmunotherapy + BEAM.39 Four patients (4/33, 12%) experienced ES a median of 8 days (range, 7-10 days) after AHCT; only 1 patient was treated with corticosteroids. Two other patients experienced febrile syndromes near the time of engraftment (day +12 and +25) that required steroids but did not meet formal criteria for ES. Twenty-two (69%) patients received post-AHCT consolidation with BV (n = 7), combination BV+PD-1 blockade (n = 14), or PD-1 blockade alone (n = 1) (supplemental Table 3). One patient experienced grade 3 hepatitis/colitis/pruritis after AHCT that was considered related to pre-AHCT Nivo on study. Another patient developed grade 2 pneumonitis post-AHCT during post-AHCT consolidation with pembrolizumab.
Among the 33 patients who proceeded to AHCT directly after protocol therapy (Nivo/NICE), all were in CR at the time of AHCT (median follow-up after AHCT of 26.2 months; range, 11.0-42.2). The 2-year post-AHCT PFS and OS among these patients were 94% (95% CI: 77-98) and 97% (95% CI: 80-99.6) (Figure 3). PFS events included 1 of 32 patients who relapsed after AHCT, and 1 patient who died of Pneumocystis jirovecii pneumonia after noncompliance with prescribed trimethoprim/sulfamethoxazole prophylaxis. The 2-year PFS after AHCT was 94% for patients who were primary refractory (n = 16) as well as for patients who relapsed (n = 16) after frontline therapy (95% CI: 63-99). The 2-year PFSs among patients who received Nivo alone (n = 25) compared with NICE (n = 7) prior to AHCT were 96% (95% CI: 76-99) and 86% (95% CI: 33-98), respectively. Patients who received consolidation had a 2-year PFS of 91% (95% CI: 69-98); patients who received no consolidation had a 2-year PFS of 100%.
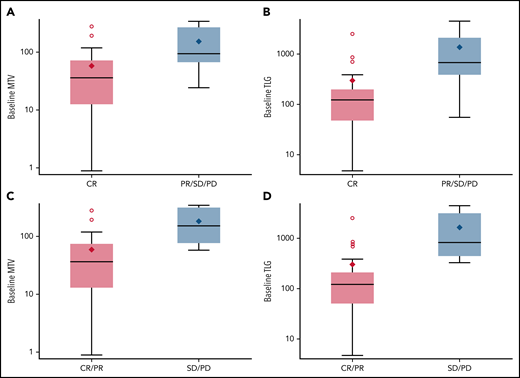
Baseline total MTV and TLG (plotted on a log scale) are associated with response status at end of Nivo. (A) Baseline MTV and CR at end of Nivo. (B) Baseline TLG and CR at end of Nivo. (C) Baseline MTV and response at end of Nivo. (D) Baseline TLG and response at end of Nivo.
Baseline total MTV and TLG (plotted on a log scale) are associated with response status at end of Nivo. (A) Baseline MTV and CR at end of Nivo. (B) Baseline TLG and CR at end of Nivo. (C) Baseline MTV and response at end of Nivo. (D) Baseline TLG and response at end of Nivo.
Nontransplanted patient outcomes
In total, 10 patients did not proceed directly to AHCT, including the 1 patient who died of sepsis during Nivo monotherapy. Two patients discontinued protocol therapy due to toxicity: 1 patient had PD and then was intolerant of BV (pneumonitis) and ultimately achieved CR with ICE and proceeded to AHCT; 1 patient received BV followed by BV+cyclosporine after PD, and then had PR to bendamustine, gemcitabine, and vinorelbine and proceeded to AHCT. One patient with PD on Nivo refused NICE, withdrew from the protocol, and remains in ongoing PR following radiation.
Four patients in CR after Nivo monotherapy refused AHCT: 3 patients discontinued Nivo, and all 3 patients had PD (1 patient had PD to Nivo retreatment and then proceeded to AHCT following PR to BV, 1 patient had CR to anti-PD-1 retreatment but again refused AHCT, 1 patient responded to several subsequent treatments but continued to refuse AHCT), 1 patient continued Nivo off-protocol and remains in ongoing remission. One patient in CR after NICE refused AHCT, experienced PD, and then responded to BV+bendamustine but continued to refuse AHCT. One patient in PR after NICE had a deeper PR to subsequent BV+cyclosporine and proceeded to AHCT, relapsed, and then ultimately proceeded to allogeneic HCT after 2 subsequent regimens. In total, 4 of the 5 patients who refused AHCT after achieving CR to protocol therapy ultimately progressed at a median of 7.5 months.
Quantitative PET assessment
Paired baseline and end of Nivo PET-CT images were available and of sufficient quality for assessment in 28 patients. The median MTV and TLG values at baseline were 51.03 (range, 0.88-340.19) and 146.76 (range, 4.7-4468.77), respectively. Baseline MTV and TLG were significantly lower in patients who achieved a CR at the end of Nivo treatment (n = 20) as compared with patients with less than CR (PR, SD, or PD) end of Nivo (n = 8) (MTV: P = .014; TLG: P = .0076). Similarly, baseline MTV and TLG were significantly lower in responding patients (CR or PR) at the end of Nivo (n = 22) as compared with nonresponders (SD or PD) (n = 6) (MTV: P = .0092; TLG: P = .0039) (Figure 3).
TME analysis
Twenty patients (14 responders to second-line Nivo, 6 nonresponders) had available tumor tissue obtained at the time of relapse/primary refractory cHL, and 11 patients had paired tumor specimens available from the time of diagnosis. In patients who did not achieve CR to second-line Nivo, there was a higher density of CD8+PD-1+ T cells (P = .0025), total CD8+ T cells (P = .0136), CD163+PD-L1+ macrophages (P = .008853), and total CD163+ macrophages in the TME at relapse prior to treatment (P = .0055) compared with complete responders (Figure 4). Nearest neighbor analysis demonstrated that among patients who did not achieve CR to second-line Nivo, there was a higher density of CD8+PD-1+ (P = .004), total CD8+ (P = .003), CD4+PD-1+ (P = .03), total CD3+ T cells (P = .039), PAX5+CD79a+ B cells (P = .0127), CD163+PD-L1+ macrophages (P < .001), CD163+PD-L1− macrophages (P = .0024), total CD163 macrophages (P < .001), and CD56+ NK cells (P = .02436) in close proximity (P values shown for 40-μm distance, but also significant at 20 μm, 30 μm, 50 μm, and 60 μm) to Hodgkin Reed-Sternberg (HRS) cells compared with CR patients (Figure 4). Analysis of paired diagnostic and relapse specimens demonstrated that at relapse, there was a lower density of CD4+PD-1+ T cells (P = .036), total CD4+ T cells (P = .01), total CD3+PD-1+ T cells (P = .047), total CD3+ T cells (P = .045), and a lower CD4:CD8 T-cell ratio (P = .025) in the TME. At relapse, the average density of PD-L1+ macrophages were higher than PD-L1+ HRS cells (P = .02). At diagnosis, the mean distance between PD-L1+CD163+ macrophages and the nearest PD-L1+ HRS was less than the mean distance between PD-L1−CD163+ macrophages to the nearest PD-L1+ HRS in diagnostic samples (P = .0008), with a similar trend in relapse samples (supplemental Figure 1). The mean distance of PD-1+ CD4 T cells to the nearest HRS cell was less than the mean distance of PD-1+ CD8 T cells to the nearest HRS cell in both diagnostic (P = .037) and relapse samples (P = .0282) (supplemental Figure 2).
Spatial features of the TME associated with CR to second-line Nivo. (A) Density of CD8+PD-1+ T cells in CR vs non-CR patients. (B) Density of CD163+PD-L1+ macrophages in CR vs non-CR patients. (C) CD8+PD-1+ T cells within 40 μm of HRS cells in CR vs non-CR patients. (D) CD163+PD-L1+ macrophages within 40 μm of HRS cells in CR vs non-CR patients. (E) CD56+ NK cells within 40 μm of HRS cells in CR vs non-CR patients.
Spatial features of the TME associated with CR to second-line Nivo. (A) Density of CD8+PD-1+ T cells in CR vs non-CR patients. (B) Density of CD163+PD-L1+ macrophages in CR vs non-CR patients. (C) CD8+PD-1+ T cells within 40 μm of HRS cells in CR vs non-CR patients. (D) CD163+PD-L1+ macrophages within 40 μm of HRS cells in CR vs non-CR patients. (E) CD56+ NK cells within 40 μm of HRS cells in CR vs non-CR patients.
Discussion
PET-adapted sequential salvage therapy with Nivo followed by NICE was well tolerated and effective, resulting in a high CR rate and allowing most patients to proceed to AHCT without the use of chemotherapy. Patients who received Nivo and then proceeded directly to AHCT in response had few relapses and excellent post-AHCT outcomes, suggesting that PD-1 blockade alone can serve as an effective bridge to AHCT. Although few patients ultimately received it, sequential NICE appeared safe after second-line Nivo with no unexpected safety signals. The CR rate to NICE was high among patients not in CR after Nivo alone, including patients who had PD with Nivo alone. Stem cell mobilization and collection were adequate following Nivo or sequential Nivo/NICE, and there were no unexpected toxicities observed after AHCT in this cohort of anti-PD-1–treated patients.
There is no consensus about the optimal salvage regimen in patients with RR cHL. Traditionally, combination chemotherapy has been the standard second-line therapy for cHL.15-20 More recently, the targeted novel immunotherapies, BV and PD-1 blockade, have been incorporated into salvage therapy with promising results.21-29,35,40 Sequential BV followed by chemotherapy was associated with a high CR rate as an overall strategy. Although only a minority of patients achieved CR with BV alone, these patients were able to proceed directly to AHCT and had similar outcomes to the rest of the cohort.21,23 BV combined with chemotherapy resulted in a higher CR rate as a first strategy but increased toxicities.24-29,41 BV+Nivo as first salvage therapy resulted in a similar CR rate and PFS to sequential or combination BV-based salvage approaches, including a 3-year PFS of 91% in patients who proceeded directly to AHCT after BV+Nivo.34,35 Notably, after BV+Nivo salvage, the CR rate and PFS in patients with primary refractory cHL was lower than what was observed in patients with relapsed cHL. Nevertheless, in our study, PET-adapted sequential Nivo/NICE yielded similar responses and PFS in patients with relapsed and primary refractory cHL. Ultimately, the relative contribution of BV vs PD-1 blockade to the efficacy of BV+Nivo is unclear. With administration of BV during frontline therapy for cHL increasing, the utility of BV-based salvage may decline, making it critical to study PD-1 blockade as part of salvage therapy independent of BV.
Similar to the introduction of BV into salvage therapy, PD-1 blockade has now been studied in sequence with chemotherapy through this study and in combination with chemotherapy in other studies. Pembrolizumab combined with gemcitabine, vinorelbine, and liposomal doxorubicin (GVD) resulted in a high CR rate after 2 or 4 cycles and was well tolerated, and thus far, no patients have relapsed after AHCT. A high rate of post-AHCT ES and immune-related post-AHCT toxicities were observed but were manageable.42 Preliminary results of a study using pembrolizumab combined with ICE suggest that the regimen is well tolerated.43 We used a PET-adapted sequential anti-PD-1/chemotherapy approach and observed a similarly high CR rate as was observed with concurrent Pembro-GVD. The durable responses after AHCT observed with longer follow-up in our study appear to confirm the promising early PFS observed after Pembro-GVD and AHCT. An advantage of our sequential approach is that most patients were able to avoid salvage chemotherapy and were successfully bridged to AHCT with PD-1 blockade alone. We also observed lower rates of post-AHCT ES and immune-related toxicity after AHCT. The excellent PFS observed following salvage Nivo monotherapy followed by AHCT challenges the traditional dogma that AHCT should be reserved for patients who are chemosensitive. Other novel salvage therapy studies (eg, BV+Nivo) either have incorporated BV, an antibody drug conjugate engineered to deliver targeted chemotherapy, or combined PD-1 blockade with chemotherapy. Our study is the first prospective study to demonstrate that sensitivity to PD-1 blockade alone can result in successful AHCT. Further work is needed to understand which patients may be suitable for a sequential as compared with a combination approach: patients with late relapse, low disease burden, older age, or more comorbidities may be good candidates for sequential therapy. A disadvantage to our sequential approach is the longer interval between initiation of salvage therapy and AHCT in the minority of patients who required NICE. Nevertheless, our results and the results of other studies using PD-1 blockade as part of salvage therapy make a compelling case for the further study of anti-PD-1–based salvage therapy.42-44
We observed a CR rate to single-agent PD-1 blockade as second-line therapy that is higher than has been previously observed in the frontline or later RR settings.30-33 This may be due to the smaller number of patients and centers in our study or at least in part due to the commonly observed differences between investigator-assessed CR rate (used in our study) and independent response assessment (performed in the pivotal studies). However, there is also the possibility that there are unique biological features about initial relapse of or primary refractory cHL that impact susceptibility to PD-1 blockade; this requires further investigation.
In our study, baseline MTV was associated with CR and response to Nivo in RR cHL, similar to what has been observed with chemotherapy or BV.45-53 With further validation of our findings, MTV may prove to be a useful tool for selecting patients for a sequential PET-adapted strategy as evaluated in this study. Patients with a higher metabolic tumor burden may prove to be better candidates for concurrent PD-1 blockade and salvage chemotherapy. MTV has been assessed in studies evaluating frontline PD-1 blockade in cHL with somewhat differing results. Baseline MTV was not predictive of response to frontline pembrolizumab in patients with cHL, although another study evaluating sequential or concurrent Nivo with AVD chemotherapy in patients with early-stage cHL reported that patients who had baseline MTV above the median were less likely to achieve CR compared with patients with MTV lower than the median.54,55 These differences may be related to the setting; our study evaluated patients with RR cHL rather than newly diagnosed patients.
In our mIF analysis of the cHL TME, we observed similar findings to previous studies,56 including a higher density of PD-L1+ macrophages than PD-L1+ HRS in the TME, a closer proximity between PD-L1+ macrophages to PD-L1+ HRS cells than PD-L1− macrophages to PD-L1+ HRS, and a closer mean distance between PD-1+CD4+ T cells and HRS cells as compared with PD-1+CD8+ T cells and HRS cells. In our cohort, certain TME features were associated with lack of CR to Nivo, including a higher density of certain cell types in the overall TME (eg, CD8+PD-1+ T cells or CD163+PD-L1+ macrophages) or a higher proportion of certain cell populations within close proximity to HRS cells (eg, CD163+PD-L1+ macrophages, CD56+ NK cells, and PD-1+ T cells). A preliminary hypothesis based on our findings may be that the action of Nivo in cHL relies at least in part on exerting influence (eg, reversing T-cell exhaustion) on local immune cells and that a more “immunosuppressive” TME (ie, more PD-1+ T cells, more PD-L1+ macrophages) may impede deeper responses to Nivo in R/R cHL. However, because of the small sample size of our analysis, it is difficult to draw firm conclusions about the R/R cHL TME and response to Nivo.
In conclusion, PET-adapted sequential Nivo/NICE salvage therapy was a safe and effective bridge to AHCT that resulted in a high rate of durable remissions in patients who proceeded to AHCT. Our findings along with the promising results observed in other studies incorporating PD-1 blockade into salvage therapy for cHL suggest that a randomized comparison of conventional vs anti-PD-1–based salvage therapy should be performed.
Acknowledgments
This research was supported by the National Institutes of Health, National Cancer Institute grant P50 CA107399-11A1, the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award (A.F.H.), and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award (A.F.H.). Bristol-Myers Squibb provided drug and funding support for this clinical trial.
Authorship
Contribution: A.F.H., R.C., J.M.P., S.J.F., and D.L.S. conceived the study design; A.F.H., J.M.P., and N.-C.T. analyzed the data; A.F.H., M.G.M., and J.M.P. prepared the first draft; M.G.M., K.M., I.M., J.Y.S., K.-J.B., S.A., M.N., P.P.L., J.Z., L.N., L.P., A.N., A.C., S.R., and L.K. collected and assembled the data, interpreted the data, and revised the manuscript; H.J.L., J.M.P., and A.F.H. designed the study, collected and assembled the data, analyzed and interpreted the data, and prepared the first draft and revised the manuscript; R.C., D.L.S., and S.J.F. designed the study, collected and assembled the data, interpreted the data, and revised the manuscript; and N.-C.T. and L.C. collected and assembled the data, analyzed and interpreted the data, and revised the manuscript.
Conflict-of-interest disclosure: A.F.H. reports research funding from BMS, Merck, Genentech, Inc/F. Hoffmann-La Roche Ltd, Gilead Sciences, Seattle Genetics, AstraZeneca, and ADC Therapeutics; consultancy for BMS, Merck, Genentech, Inc/F. Hoffmann-La Roche Ltd, Kite Pharma/Gilead, Seattle Genetics, Karyopharm, Takeda, Tubulis, AstraZeneca, Genmab, ADC Therapeutics, and Regeneron. M.G.M. reports research funding from TG Therapeutics, Epizyme, and BMS, and consultancy with Morphosys and GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Alex F. Herrera, City of Hope National Medical Center, City of Hope, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: aherrera@coh.org.
Individual participant data will not be shared.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
M.G.M. and H.J.L. contributed equally to this study.