Abstract
Immunotherapies have recently shown efficacy in treatment of aggressive, refractory pediatric B cell acute lymphoblastic leukemia (B-ALL), which remains one of the leading causes of cancer-related death in children. The immune evasion mechanisms of B-ALL are still being explored to discover new therapeutic targets and improve patient outcomes.
Recent reports have implicated a role for the molecule Siglec-15 (Sig15) in regulating immune response in solid tumor-infiltrating macrophages. Our lab has found higher expression of SIGLEC15 at the RNA level in primary pediatric B-ALL as compared to healthy donor controls, as well as at the RNA and protein levels across a panel of B-ALL, T cell acute lymphoblastic leukemia (T-ALL), and diffuse large B cell lymphoma (DLBCL) cell lines compared to healthy donor PBMCs. Higher expression of SIGLEC15 in pediatric B-ALL samples from the TARGET database correlates with markers of PKC and NFκB activation known to drive B-ALL leukemogenesis, which we have demonstrated to regulate Sig15 RNA and protein expression in vitro.
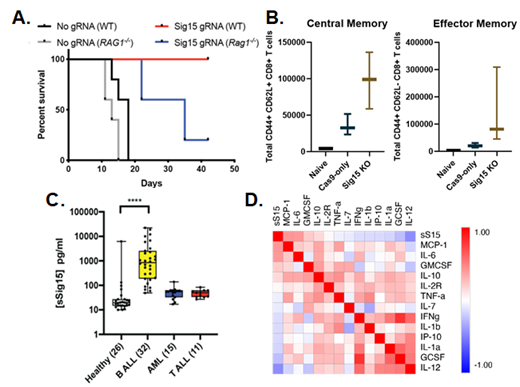
Knockout of Siglec15 expression in a BCR-ABL1 + murine model of B-ALL engrafted in immunocompetent and Rag1 -/- immunodeficient recipients resulted in leukemia clearance in immunocompetent, but not immunodeficient, recipients and 100% survival (Figure A, p=0.01 Sig15 KO into WT vs. Rag1 -/-). Further study indicates that Siglec15 expression on these leukemia cells suppresses T cell effector and memory population expansion at 7 days post-engraftment (Figure B) and correlates with higher levels of IL-10 and lower levels of CCL17 present in the bone marrow, representing a more immunosuppressive bone marrow milieu. These data suggest a prominent role for Sig15 in the suppression of adaptive immune response to B-ALL as well as other hematological malignancies.
We have also reported for the first time the release of a soluble form of Sig15 (sSig15), which we have demonstrated to circulate at higher levels in the plasma of pediatric B-ALL patients compared to healthy donors (Figure C, ****P≤0.0001). Detection of this sSig15 negatively correlated with circulating levels of IL-12 and IL-1α/β (Figure D, depicting correlations of cytokines using Pearson's r), suggesting sSig15 levels correspond to a systemically immunosuppressive phenotype. Flow cytometry of fresh pediatric B ALL cells demonstrates expression of surface Sig15 in a subset of cases. Thus, Sig15 has the capacity to promote immunosuppressive effects at both marrow-localized and systemic levels.
Together, these results suggest Siglec-15 is a novel, potent immunosuppressive molecule active in leukemia progression that may be targeted therapeutically to activate T lymphocytes against leukemia cells.
Abukharma: NextCure Inc.: Current Employment. Liu: NextCure: Current Employment, Current holder of stock options in a privately-held company.