Abstract
Background: CML is managed by life-long TKI therapy in most pts which can be associated with chronic toxicities and substantial costs. Discontinuation of TKI in some pts can result in sustained therapy-free remission (TFR); however only a minority are candidates for discontinuation approaches; of those most still relapse and must restart TKI. High expression of programmed death receptor-1 (PD-1) and its ligand PD-L1 on CML-specific cytotoxic T-cells and CML cells, respectively, suggests that PD-1/PD-L1-mediated immune escape contribute to CML persistence and relapse after TKI discontinuation. We designed the BLAST MRD CML trial (NCT#03516279) to study whether adding anti-PD-1 antibody pembrolizumab to TKI therapy is safe and tolerable and increases rate of conversion to undetectable minimal residual disease (UMRD). We present data from the early safety cohort from this ongoing trial with a data cut-off date June 15th, 2021.
Methods: BLAST MRD CML-1 is an ongoing national, ECOG-sponsored, single-arm pilot phase II clinical trial that enrolls adults with chronic phase CML who achieved a major molecular response (MR 3) but not UMRD while on a stable dose of up to three lines of TKI therapy. UMRD was defined as an undetectable BCR-ABL by central RQ-PCR (assay sensitivity of 4.5 [MR 4.5]) on 2 consecutive samples separated by ≥4 weeks. Once enrolled, pts continue on TKI and concurrently receive pembrolizumab 200mg IV on day (D)1 of each 21-D cycle with central MRD assessment every 4 cycles for 17 cycles. Pts who achieve UMRD at or prior to C17 discontinue pembrolizumab, while those with persistent MRD continue TKI + pembrolizumab for another 18 cycles. TKI is discontinued one year following first documentation of UMRD. After TKI discontinuation, BCR-ABL is monitored at defined intervals for 24 months. Primary endpoint is proportion of CML pts on stable-dose TKI who convert to UMRD within 2 years (yrs) of combined TKI + pembrolizumab therapy. Secondary endpoints include rates of TKI discontinuation and proportion of pts achieving 2-year TFR, MRD kinetics, and safety. Immune related and other adverse events (AE) were assessed and graded according to CTCAE v 5.0.
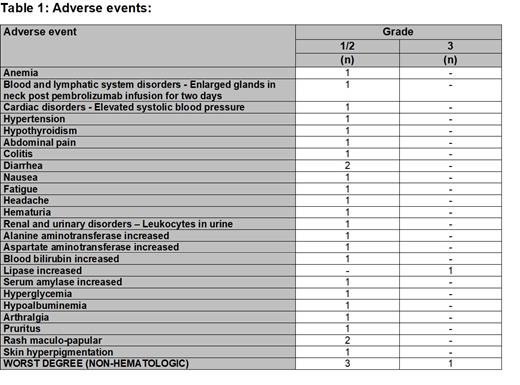
Results: As of cut-off date, 6 pts enrolled in safety cohort. Median age was 43 yrs (range 32-65). TKIs received on trial included imatinib (N=2), dasatinib (N=3), and nilotinib (N=1). All 6 pts were on 2 nd line TKI. Median exposure to pembrolizumab was 20 doses (range, 5-36). Five pts were still on combined pembrolizumab + TKI treatment, while the 6 th pt has completed combined therapy for 2 yrs and then continued TKI monotherapy on trial after achievement of UMRD (pt has to be in UMRD for 1 yr before TKI can be discontinued). The combination of TKI and pembrolizumab was safe and well tolerated (Table 1). Only 3 pts experienced grade (G) 1/2 AEs as the worst non-hematologic AE while on trial. Hematologic toxicity reported was also generally minimal with only one G 1/2 anemia AE. The only reported G3 AE was a lipase elevation (283 U/L, normal range 11 - 55 U/L) noted on routine laboratory assessment in a pt receiving nilotinib at 400mg BID dose after 48 weeks of starting on trial combined therapy. Amylase was also elevated at G2. No baseline lipase levels were available as protocol at that time did not require baseline assessments. The pt had no symptoms and CT imaging showed no radiologic evidence of pancreatitis. The lipase and amylase levels normalized with holding of both nilotinib and pembrolizumab for 7 weeks, and the AE did not recur with resumption of combined therapy at same dose of pembrolizumab but with nilotinib reduced to 400mg daily. No G4 or 5 AEs occurred. No other dose-adjustments or treatment discontinuation/interruptions due to AEs occurred. No immune-related AEs (IRAEs) have been reported.
Discussion: Data from the early safety cohort suggest that the combination of TKI and pembrolizumab is safe and well tolerated. The AEs observed were mild and generally G1/2. No pt discontinued trial therapy due to AEs. Only one G3 AE (asymptomatic lipase elevation) was reported, which could have been related to nilotinib therapy and could have predated trial entry as no baseline lipase value was available and was fully reversible with a temporary hold of therapy. No IRAEs have been reported to date. Work is ongoing on an amendment to study protocol to enhance accrual. Efficacy results will be reported as they mature.
Zeidan: Boehringer Ingelheim: Consultancy, Research Funding; Ionis: Consultancy; Gilead: Consultancy, Other: Clinical Trial Committees; Loxo Oncology: Consultancy, Other: Clinical Trial Committees; Daiichi Sankyo: Consultancy; Incyte: Consultancy, Research Funding; Astex: Research Funding; Jasper: Consultancy; Cardiff Oncology: Consultancy, Other: Travel support, Research Funding; Aprea: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; BeyondSpring: Consultancy; BMS: Consultancy, Other: Clinical Trial Committees, Research Funding; Acceleron: Consultancy, Research Funding; Novartis: Consultancy, Other: Clinical Trial Committees, Travel support, Research Funding; Genentech: Consultancy; Epizyme: Consultancy; AstraZeneca: Consultancy; BioCryst: Other: Clinical Trial Committees; Janssen: Consultancy; Kura: Consultancy, Other: Clinical Trial Committees; Geron: Other: Clinical Trial Committees; Astellas: Consultancy; ADC Therapeutics: Research Funding; Agios: Consultancy; Jazz: Consultancy; Pfizer: Other: Travel support, Research Funding; AbbVie: Consultancy, Other: Clinical Trial Committees, Research Funding. Radich: Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Bhatt: National Marrow Donor Program: Research Funding; Tolero Pharmaceuticals, Inc: Research Funding; Pfizer: Research Funding; Incyte: Consultancy, Research Funding; Jazz: Research Funding; Abbvie: Consultancy, Research Funding; Partnership for health analytic research, LLC: Consultancy; Servier Pharmaceuticals LLC: Consultancy; Rigel: Consultancy; Abbvie: Consultancy, Research Funding; Genentech: Consultancy. Luger: Syros: Honoraria; Agios: Honoraria; Daiichi Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria; Brystol Myers Squibb: Honoraria; Acceleron: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Onconova: Research Funding; Celgene: Research Funding; Biosight: Research Funding; Hoffman LaRoche: Research Funding; Kura: Research Funding. Litzow: Omeros: Other: Advisory Board; Astellas: Research Funding; Jazz: Other: Advisory Board; Amgen: Research Funding; Pluristem: Research Funding; Biosight: Other: Data monitoring committee; Actinium: Research Funding; AbbVie: Research Funding.
Pembrolizumab has not been approved for the treatment of CML