Abstract
Background
Extensive protein synthesis in multiple myeloma (MM) cells renders them vulnerable to proteasome inhibitors (PI), a cornerstone of anti-myeloma therapy. However, relapse is inevitable and PI resistance remains a major barrier to improving outcomes. Adaptive responses to PI include upregulation of autophagy, another major degradation pathway in cells. We hypothesised that autophagy inhibition increases cell death and impedes recovery of MM cells exposed to Carfilzomib (K), a second generation PI effective in newly diagnosed and relapsed/refractory MM.
Methods:
Human myeloma cell lines (HMCL) and patient samples were exposed to a 1-hour pulse of K to mimic pharmacokinetics, ± autophagy inhibitors (AI) vacuolar protein sorting kinase 34 inhibitor (VPS34i) or unc-51 like autophagy initiating kinase inhibitor (ULKi). Apoptosis was assessed by Annexin V/Propidium iodide (AnV/PI) flow cytometry (FC) and autophagic flux by western blotting (WB) and FC for light chain 3B (LC3B) ± bafilomycin (B). Cell cycle was examined by FC using KI67/PI.
Results:
Basal autophagic flux was seen in all HMCL (MM1s, H929, KMS12BM) tested (±B). Autophagic flux increased in response to K: MM1s mean fluorescence intensity (MFI) control (CTL) 318±28 vs K 567±72, (mean±SEM),p=0.01, n=6). Autophagy was inhibited in all 3 HMCL with AI. Combined exposure to K and AI (VPS34i or ULKi) increased time dependent apoptosis in all HMCL tested: MM1s 31±8% (K) vs 58±9% (K+VPS34i), p=0.03, (n=3), and 13±0.4% (K) vs 63±16% (K+ULKi), p=0.03 (n=3), (K 10nM, AI 1uM) 48 hours(h) post K exposure.
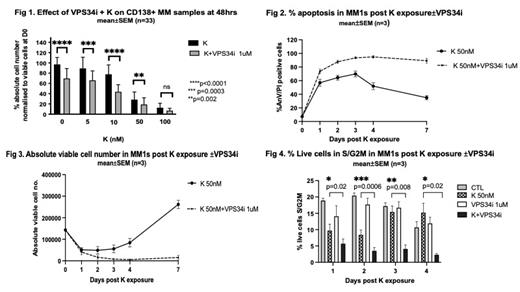
Basal autophagic flux was detected in all CD138+ primary MM patient samples tested by LC3B FC assay (MFI CTL B- 407±57 vs B+ 1081±125, p<0.0001, n=14). This increased in response to K: CTL B- 407±57 vs K B- 862±224, p=0.03, n=14. VPS34i alone reduced viable cell numbers in 26/33 primary samples (% viable cells relative to starting viable MM cell number at day 0: CTL 99±14% vs VPS34i 71±12%, p<0.0001 at 48h), and cell loss correlated with basal autophagic flux (r=0.9, Pearson's correlation coefficient, p=0.0003, n=10). Addition of VPS34i to K increased loss of viable CD138+ cells (n=33) (Fig 1). Both apoptosis with K and enhanced cell death with K+VPS34i correlated with basal autophagic flux (r=0.8, p=0.0005 and r=0.8, p<0.0001 respectively), but no correlation was seen with previous therapy, disease stage, or adverse risk by cytogenetics or ISS stage.
K treated MM1s cells were examined for kinetics of ubiquitinated protein build up and autophagic induction by WB. Increased ubiquitinated proteins were detectable up to 24h post K pulse, whilst autophagic flux increased up to 72h but returned to baseline thereafter (n=3). MM1s cells were treated with K±VPS34i for up to 7 days and apoptosis, absolute cell number and cell cycle assessed. In K treated cells, apoptosis was maximal at 72h but declined thereafter. Viable cell numbers fell initially but increased after 96h, indicating cell recovery. However, in MM1s cells treated with K+VPS34i/ULKi, apoptosis was enhanced with a rapid loss of viable cells (Fig 2) and viable cell numbers remained low at all timepoints (Fig 3). Cell cycle assays confirmed persistent G0/G1 arrest in MM1s cells treated with K+VPS34i or ULKi at all timepoints, whilst cell proliferation recovered in K treated cells on day 3, (15±3% cells in S/G2M in K treated cells vs 2±0.4% with K+VPS34i, p=0.02, n=4, 11±3% in S/G2M with K vs 1± 0.7% with K+ULKi, p=0.04, n=3, day 4)(Fig 4). To confirm that effects of AI were mediated via autophagy, KMS28PE (ATG12 null HMCL) were treated with K±VPS34i. These autophagy depleted cells showed limited basal or K induced autophagic flux and addition of VPS34i to K had no effect on apoptosis or cell cycle.
Conclusion
MM cells displayed active autophagic flux that increased with K treatment. Inhibition of autophagy increased K induced apoptosis, prolonged K-induced cell cycle arrest and prevented MM cell recovery. Addition of AI to K increased cytotoxicity in patient samples and many patients showed sensitivity to AI alone; these effects correlated with basal autophagic flux. These data suggest that autophagy plays a vital role in cell recovery from proteasome inhibition independent of proteotoxic stress. Dual inhibition of the proteasome and autophagosome may therefore help overcome PI resistance, which remains an unmet need in MM.
Auner: Takeda, Karyopharm: Other: Advisory role; Amgen: Research Funding; Janssen: Speakers Bureau. Khwaja: Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Yong: Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria; GSK: Honoraria; Amgen: Honoraria; BMS: Research Funding; Autolus: Research Funding.