In this issue of Blood, Gooptu et al and the Center for International Blood and Marrow Transplant Research (CIBMTR) have tried to answer a compelling question: what is the outcome with triple posttransplant cyclophosphamide (PTCY) prophylaxis used in matched unrelated donor (MUD) transplants, as compared with haploidentical (Haplo) grafts?1
High-dose PTCY was developed by the Baltimore group over a decade ago2 to prevent graft-versus-host disease (GVHD) in patients undergoing HLA-Haplo hematopoietic cell transplantation. The original prophylaxis included high-dose PTCY, followed by a calcineurin inhibitor (CNI) and mycophenolate mofetil.2 The advantages of high-dose PTCY can be summarized as easy, unexpensive, and very effective; thus, it is no surprise that the PTCY revolution has made Haplo transplants very popular worldwide.3-5 Now, if PTCY is so effective in patients who received grafts from Haplo donors, then why not also use it in patients who received grafts from matched unrelated donors (MUDs), in whom we still see significant acute and chronic GVHD, when using standard GVHD prophylaxis.
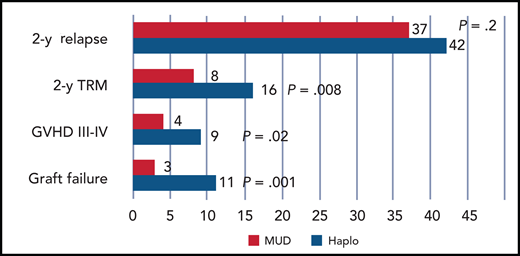
Significant reduction of graft failure, acute GVHD, and TRM in patients. Red indicates those prepared with a reduced-intensity conditioning regimen receiving an HLA MUD, as compared with a Haplo transplant, which is shown in blue. Relapse is unaffected.
Significant reduction of graft failure, acute GVHD, and TRM in patients. Red indicates those prepared with a reduced-intensity conditioning regimen receiving an HLA MUD, as compared with a Haplo transplant, which is shown in blue. Relapse is unaffected.
Acute and chronic GVHD are expected to be reduced in HLA-matched as compared with HLA-mismatched grafts when using triple PTCY-based prophylaxis. This is an important detail because PTCY has been used as a single agent in HLA-matched transplants with acceptable outcome with bone marrow as the stem cell source,6 but significant GVHD with granulocyte colony-stimulating factor–mobilized peripheral blood.7 A registry-based study by The European Society for Blood and Marrow Transplantation (EBMT) on HLA-matched grafts has shown that GVHD and relapse-free survival are best when PTCY is used together with 2 other immunosuppressive agents.8 This is the first message: the original triple PTCY combination is required for best control of GVHD in the HLA-matched setting.
In their study, Gooptu and coworkers confirm a reassuring role of HLA in predicting GVHD: patients who received grafts from HLA-matched donors had a significant reduction of both acute and chronic GVHD, and this translated into a reduction of transplant-related mortality (TRM) in patients receiving reduced-intensity regimens (see figure). In previous studies, Haplo transplants with PTCY were compared with MUD transplants receiving CNI and methotrexate for GVHD prophylaxis,9 and Haplo transplants were found to have less acute and chronic GVHD. This, of course, was comparing apples to oranges. PTCY was given only in the Haplo grafts, and donor type and HLA matching could not be evaluated given the study design. Therefore, comparing outcomes can be misleading if the starting conditions are not the same. Using consistent triple PTCY-based prophylaxis, we learn that GVHD is reduced in HLA-matched grafts as compared with HLA-mismatched grafts (see figure), and this is in line with half a century of biological and clinical studies.
Reduction of GVHD usually translates into an increased risk of relapse, due to reduced graft-versus-leukemia. One key finding of the Gooptu study is that relapse is not increased in HLA-matched grafts receiving intensive PTCY GVHD prophylaxis. This is in keeping with a previous study by the Blood and Marrow Transplant Clinical Trial Network, which compared triple PTCY-based prophylaxis to a control group of patients receiving a CNI and methotrexate regimen10 : GVHD was reduced with the triple PTCY prophylaxis and relapse again was not affected.10 A recent retrospective study by the EBMT has compared MUD and Haplo grafts in acute myeloid leukemia patients in first complete remission receiving PTCY-based prophylaxis11 : again, GVHD and TRM were increased in Haplo grafts (hazard ratio [HR], 1.6 and 2.6, respectively), but relapse was reduced (HR, 0.7) as compared with MUD transplants, which translated into comparable leukemia-free survival.
The role of PTCY in HLA-identical transplants is rapidly expanding, due to better control of GVHD compared with conventional CNI-methotrexate GVHD prophylaxis. For malignant diseases, prospective randomized trials will be needed to assess whether a significant reduction in acute and chronic GVHD will come with an increased risk of relapse. Conversely, in nonmalignant disease, where relapse is not a problem, triple or quadruple (including antithymocyte globulin) PTCY prophylaxis is becoming very popular. The PTCY Baltimore revolution in Haplo transplants is now moving to HLA-identical transplants, and may well become the standard of care in the coming years.
Conflict-of-interest disclosure: The author declares no competing financial interests.