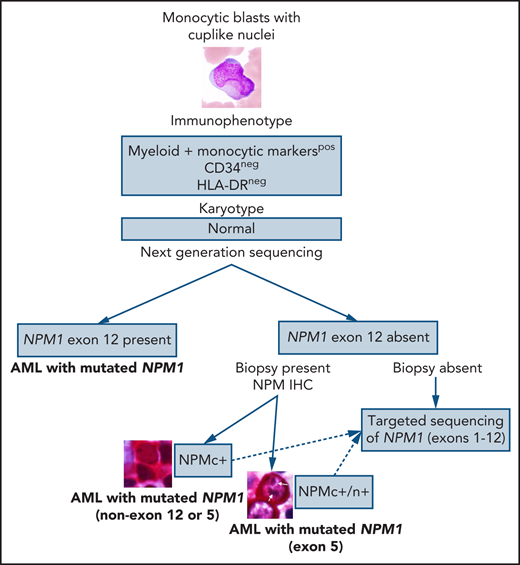
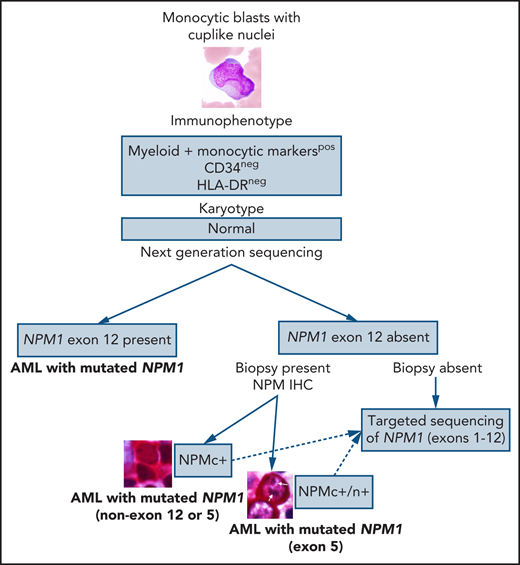
In this issue of Blood, Martelli et al describe novel exon 5 nucleophosmin (NPM1) mutations and NPM1 fusions in a large cohort of patients with acute myeloid leukemia (AML) that were discovered based on the presence of cytoplasmic NPM1 (NPM1c+) immunohistochemistry (IHC) staining in the absence of exon 12 NPM1 mutations.1
In 2017 AML with mutated NPM1 was recognized as a distinct entity by the World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Neoplasms, 1 of the 2 AML entities defined by a single gene mutation.2NPM1 is the most commonly mutated gene and is present in ∼30% of adult AML. NPM1 mutations were discovered in 2005 using IHC, long before next-generation sequencing became widely available, using the mutant protein’s unique feature of relocating from the nucleolus to the cytoplasm.3 Molecular analyses have shown that the NPM1 mutations are almost exclusively restricted to exon 12 (NM_001355006 transcript) or, alternatively annotated as exon 11 (NM_002520 transcript), and usually consist of 4-bp insertions that cause a frameshift in the last few C-terminal amino acids of the protein leading to generation of an extra nuclear export signal motif at the C terminus and cytoplasmic relocation of the protein.4 Type A mutation consists of a duplication of the TCTG coding nucleotides at codon Trp288 (p.W288fs* c.863_864dupTCTG) and is present in ∼80% of cases, followed by type B (p.W288fs* c.863_864insCATG) present in ∼10% of cases and type D (p.W288fs* c.863_864insCCTG) present in <10% of cases. Together, the presence of any of these mutations leads to an AML with mutated NPM1 diagnosis in >95% of cases and, as a result, in clinical practice sequencing of the NPM1 gene is predominantly restricted to exon 12. In addition, rarely, NPM1 translocations with novel fusion proteins are discovered by conventional karyotype translocations.
In the current era of personalized medicine and numerous (successful) attempts to develop precise diagnoses with risk stratifications and targeted therapies based on the genetic data, a question arises: Is the detection rate of the NPM1 mutation at ∼95% good enough for the diagnosis or should we do better? Several clinically significant facts point toward the latter: (1) NPM1 defines the largest AML category. (2) NPM1 mutation is present in ∼60% of normal karyotype AML. (3) NPM1 mutations almost always cooccur with other mutations, such as DNA methylation pathway-related genes or FLT3. (4) NPM1 mutations are prognostically important. (5) NPM1 can be a useful target for measurable disease monitoring. And this is when the good old-fashioned IHC comes to the rescue! The study by Martelli et al demonstrated that NPM IHC staining could be used to identify rare nonexon 12 NPM1 mutations in AML. Screening of a cohort of 929 unselected patients with AML with NPM IHC led to detection of 5 NPMc+ cases, negative for NPM1 exon 12 mutations, that on a subsequent sequencing analysis showed exon 9 (n = 1), exon 11 (n = 1), and exon 5 (n = 3) NPM1 mutations with a frequency of 0.5% of all AML cases and 1.3% of AML with mutated NPM1. An additional NPM1 exon 5 mutation was identified via targeted sequencing of exons 1 through 12; unfortunately, IHC was not available for this case because no biopsy was performed but presumably it would have been positive. All 4 NPM1 exon 5 mutations were novel and 3 of them retained the nucleolar localization signal, a unique finding, which resulted in both the cytoplasmic and nucleolar NPM staining patterns, in contrast to the cytoplasmic pattern only with exon 12 mutations.
This study once again demonstrates the importance of a holistic approach to diagnosing AML (or any cancer for that matter). The first steps in the diagnostic process of AML are evaluation of blast morphology followed by flow cytometry immunophenotyping of peripheral blood or bone marrow aspirate that could provide clues to the underlying genetic abnormalities; this morphology/immunophenotype approach is widely used for cases with suspected acute promyelocytic leukemia, allowing for rapid use of all-trans-retinoid acid to prevent disseminated intravascular coagulation and should certainly be extended to other AML categories. Monocytic or myelomonocytic morphology of the blasts with cuplike nuclear invaginations is strongly associated with the presence of NPM1 and FLT3 mutations.5 Multiple studies have shown that, in addition to frequent expression of monocytic markers, NPM1-mutated AML is predominantly negative or dim for CD34.4 Another recent study expanded on this immunophenotype/genetic correlation demonstrating that AML with mutated NPM1 with CD34−/HLA-DR−/CD117+/MPObright immunophenotype is associated with TET2 or IDH1/2 mutations with a sensitivity of 70% and specificity of 96%.6 These morphologic and immunophenotypic clues could help suspect a diagnosis of AML with mutated NPM1 even before molecular analyses are performed and should definitely prompt NPM IHC in cases where NPM1 mutation has not been detected by sequencing (see figure). Yes, these novel NPM1 mutations constitute less than 1% of all AML, but only by making an effort to detect them, we will be able to assess their clinical significance. And it looks like NPM IHC is up for the job!
Conflict-of-interest disclosure: The author declares no competing financial interests.