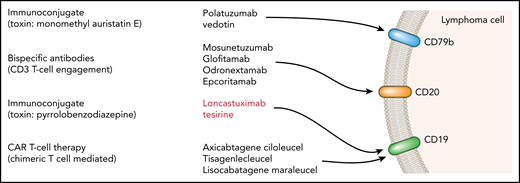
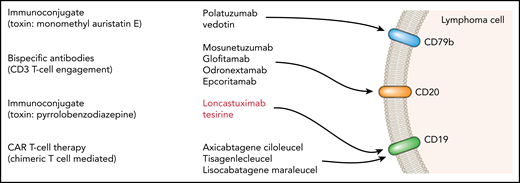
Schematic summary of a range of antibody-targeted therapies in lymphoma.
Schematic summary of a range of antibody-targeted therapies in lymphoma.
Military analogies are commonly used when describing immunoconjugate therapies, and the use of the word warhead is noticeable in the report by Hamadani et al in this issue of Blood. The authors conducted a 2-part dose-escalation/dose-expansion phase 1 study of the use of loncastuximab tesirine in relapsed non-Hodgkin lymphoma (NHL) of a range of histological subtypes.1
The efficacy that they report looks encouraging for patients with diffuse large B-cell lymphoma (DLBCL), who were by far the biggest subgroup in the study. DLBCL patients probably also represent the area of greatest unmet need among participants in the study. The overall response rate reported in evaluable DLBCL patients was 42.3%, and there was a complete response rate of 23.4%. Kaplan-Meier plots provide further support, with evidence of good duration of response in some patients.
An important exclusion criterion in the study was that patients not have another option for treatment of B-cell NHL at their current state of disease. It is notable that the timing of study recruitment (March 2016 to May 2018) meant that chimeric antigen receptor (CAR) T-cell therapy was not widely available; therefore, only a small number of patients were included as post–CAR T-cell recipients. The toxicities reported were considerable, with thrombocytopenia, edema, and fatigue all being prominent. The observation of these toxicities is important, not only for consideration of single-agent tolerability, but also for potential planning of future combination studies using this agent.
The study was designed as a standard (3 + 3) phase 1 dose-escalation/dose-expansion study, with dose-limiting toxicities, as is usual, defined during cycle 1. However, although the maximum tolerated dose (MTD) was not reached even at 200 μg/kg, it was clear that cumulative toxicity (especially thrombocytopenia) was important, particularly at the higher-dose levels. This meant that the 120 and 150 μg/kg dose levels, not the highest dose level of 200 μg/kg, were chosen for the part 2 dose-expansion cohort, even though the MTD had not been reached. In addition, there was provision in part 2 for a dose reduction after 3 cycles. At the end of both parts 1 and 2 of the study, the chosen dose schedule for the phase 2 study was 2 cycles at 150 μg/kg followed by subsequent cycles at 75 μg/kg. The rationale for this choice is well presented by the authors, who tried to overcome the challenge of using a conventional dose-finding study design with a novel targeted agent. It is clear that cumulative toxicity was particularly important, with the half-life of the conjugated antibody at the 150 μg/kg dose level noted to double between the first and second cycles. The cumulative toxicity observed, especially in the highest-dose cohort, led to some dose delays being necessary. This drug accumulation as cycles are delivered might be in part related to the decreasing availability of cell membrane targets when tumor response occurs.
The difficulty of these complex pharmacokinetic considerations and the consequent regimen adjustment is that data on response and toxicity with the specific dose schedule chosen for the planned phase 2 study have not been tested in this phase 1 study. The schedule chosen represents an intelligent emergent strategy to find the optimal dosing schedule achieved by reacting to the observed cumulative toxicity seen in the first part of the study. The supplemental data available, as ever, merits detailed scrutiny for the best understanding of the main article. This difficulty illustrates the need for differences in strategy when developing novel agents compared with traditional dose finding in conventional chemotherapy. The importance of this observation for study design can be seen to go well beyond this particular study, although the possible options for addressing this are beyond the scope of this commentary.
The key question remaining is where in the treatment pathway this agent can be placed. CAR T-cell therapy (currently with axicabtagene ciloleucel2 and tisagenlecleucel3 ) is rapidly becoming established as a treatment of choice, in those fit enough, for the management of DLBCL which has relapsed after 2 lines of therapy. In those patients with insufficient performance status or who have already received CAR T cells, the combinations of polatuzumab vedotin and bendamustine4 or tafasitamab with lenalidomide5 are available, but many other novel targeted agents are in current clinical trials6-9 (see figure for an illustrative but not comprehensive list).
Some new agents, used in combination, may move earlier in the treatment pathway, thus leaving a niche at relapse. It may be difficult to find a place for loncastuximab teserine earlier or in combination with chemotherapy because of the toxicities reported by Hamadani et al (although it should be noted that there are examples of combination schedules with other novel agents referenced in the report, such as the study registered at www.clinicaltrials.gov as #NCT03684694). Lastly, it is possible that tumor cells, under selection pressure, may lose a targeted antigen to escape from therapeutic control. Loncastuximab tesirine shares the CD19 target with current CAR T-cell products, and this might be seen as an argument for its use after CAR T-cell therapy or in patients unsuitable for it.
In conclusion, these results suggest that the data from the subsequent phase 2 study of loncastuximab tesirine will be awaited with interest. Great hope has been invested in CAR T-cell therapy, but it is likely there will remain an unmet need for patients after CAR T-cell failure, and it seems most likely that this is where loncastuximab tesirine may find a role.
Conflict-of-interest disclosure: A.M. serves on speakers bureaus, is a member of advisory boards, and receives travel sponsorship for Roche and Celgene.