The coronavirus disease 2019 (COVID-19) outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic on March 11, 2020. This novel virus can cause a rapid progression from cough to acute respiratory distress syndrome and death. Cancer patients infected with COVID-19 were reported to have a 39% incidence of severe events, including admission to intensive care unit (ICU), mechanical ventilation, or death. Patients with hematologic malignancies, especially those undergoing treatment, are a particularly at-risk population due to disease-related impairment of the immune system and chemotherapy-induced neutropenia. The goal of this study is to analyze outcomes of COVID-19 infected patients with hematologic malignancies in order to better understand the impact of SARS-CoV-2 on this vulnerable population.
Methods
We performed a retrospective analysis on 26 COVID-19 positive patients with hematologic malignancies identified at our center. On July 22, 2020, there were 264 COVID-19 positive patients with hematologic malignancies (including our center's 26 patients) reported to the American Society of Hematology Research Collaborative COVID-19 Registry (ASH RC), a global public reference tool. We extracted our patient's data from each category reported to the ASH RC and compared hospitalization, ICU admissions, and mortality rates between our cohort and the remaining 238 cases. Chi-square test was used for analyses. We also performed a subgroup analysis comparing demographics; type and status of hematologic malignancy, as well as COVID-19 directed treatments between our center's patients and the patients reported to the ASH RC.
Results
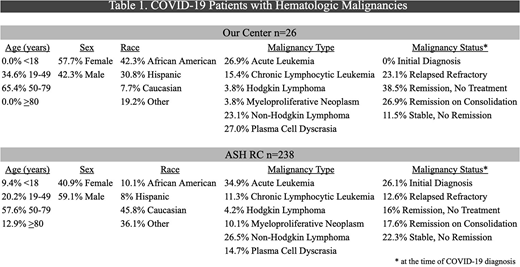
Between March and June 2020, a total of 1265 COVID-19 positive patients were hospitalized at our institution. A significantly higher percentage of COVID-19 patients with hematologic malignancies were hospitalized at our institution compared to the ASH RC (61.5% versus 35.3%, P=. 009). There was no difference in ICU admission rate at our center compared to the ASH RC (23.1% versus 30.2%, P=.45). Significantly less COVID-19 directed therapies were administered at our center compared to the ASH RC (46.2% versus 66.4%, P=.041). Our patients received: 7.7% Remdesivir, 11.5% Tocilizumab, 15.4% hydroxychloroquine, 11.5% azithromycin, 0% convalescent plasma, compared to the ASH RC: 1.7% Remdesivir, 5.5% Tocilizumab, 30.3% hydroxychloroquine, 25.6% azithromycin, 4.6% convalescent plasma. Lastly, our institution had a significantly decreased mortality rate compared to the ASH RC (11.5% versus 29.8%, P=.049). Demographics as well as type and status of hematologic malignancy comparing the two cohorts are shown in Table 1.
Conclusions
In our comparative analysis, we found that our center's patients were hospitalized significantly more than the ASH RC cohort yet had lower mortality rates. These differences were seen despite similar distribution of malignancy types between the two groups. It should be noted that more patients in our cohort were in remission and none presented at initial cancer diagnosis at the time of infection, which may have contributed to better outcomes. The difference in mortality rates may also be attributed to variance in provider experience, higher percentage of patients >80 years of age reported to the ASH RC, and closer patient monitoring at our center due to a higher hospitalization rate. Differences in ICU admissions were not significant, suggesting a similar rate of severe COVID-19 infection between the two cohorts. Our demographics reflect the urban population we serve with more African Americans and Hispanics compared to the ASH RC. The greater number of COVID-19 directed therapies in the ASH RC cohort compared to ours is likely attributed to the use of convalescent plasma, which was not commonly used as COVID-19 directed treatment at our institution. Limitations of our study include a restricted time frame, small sample size, and the possibility of incomplete datasets within the ASH RC, as stated on the registry's website. In conclusion, we recommend close monitoring and a lower threshold for hospitalizing patients with hematologic malignancies in the setting of COVID-19 infection; however, additional prospective studies are needed to confirm our findings, and further investigate the complications and outcomes of SARS-CoV-2 on this at-risk population.
Ustun:Kadmon: Honoraria. Shammo:Celgene: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; Onconova: Research Funding; Incyte: Consultancy, Research Funding, Speakers Bureau; Apellis: Consultancy; Regeneron: Consultancy; Novartis: Consultancy; Agios: Consultancy; Sanofi: Speakers Bureau; Abbive: Current equity holder in publicly-traded company; Baxter: Current equity holder in publicly-traded company; Takeda: Current equity holder in publicly-traded company; Alexion: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.