Abstract
Anemia is a common finding in the perioperative setting with significant untoward consequences including worsening of outcomes and diminished quality of life as well as increased risk of allogeneic blood transfusions. Here, we present 3 cases that illustrate how anemia can be perioperatively managed in patients undergoing cardiac, orthopedic, and oncology surgeries. Timely detection of anemia prior to high-blood loss surgeries can allow clinicians to manage it and optimize hemoglobin level, making patients better prepared for the surgery. Treatment of anemia should be guided by the etiology and may include erythropoietic agents, folic acid, B12, and iron preparations. Other blood management strategies geared toward reducing surgical blood loss such as autologous transfusion techniques and agents to optimize hemostasis are used during surgery and in the immediate postoperative period. Patients should be closely monitored following surgery for signs of ongoing bleeding in need of control. Finally, screening for and management of anemia should continue in the postoperative and postdischarge period, as persistence and recurrence of anemia can further undermine patient’s outcomes.
Introduction
Although accounts of anemia date back several centuries, Thomas Sydenham (1624-1689) is among the first physicians to be credited with the successful treatment of the condition with “steel filings steeped in cold Rhenish wine." Not to undermine the delightful value of an occasional glass of good wine, he was quick to point out that his remedy was essentially iron in liquid form.1(p727)
Anemia remains a common condition across the globe, with particular ramifications in the perisurgical setting. It is generally reported to be present in 10% to 50% of the patients in the preoperative setting and as much as 90% of the patients in the postoperative setting.2,3 Its highly frequent and seemingly low-profile presence should not be taken as an indicator of a benign nature. Perioperative anemia is an independent risk factor for mortality and morbidity, and anemic patients commonly endure slowed recovery, prolonged hospital stay, and diminished quality of life.3-6 Even mild to moderate anemia can have a detrimental impact on patient outcomes. According to a large study report in over 300 000 veterans, the adjusted risk of 30-day postoperative mortality and cardiac morbidity begins to increase as the hematocrit declines from 39, exposing even those with mild anemia to additional risks.6,7
Availability of allogeneic blood transfusions has arguably contributed to a false sense of security among clinicians. Long considered to be the default treatment, it is hard to argue against the apparent ease of ordering and administering blood compared with the relatively tedious, etiology-based approach to managing anemia. This view is quickly shattered when the long list of risks and complications associated with transfusion is considered. In addition to recognized risks such as transfusion errors and transfusion-transmitted infections, patients who are transfused are often at increased risk of mortality and morbidity, namely various organ failures, infections and thromboembolic events.8,9 Intraoperative transfusion has been shown to independently increase the risk of sepsis as well as pulmonary, thromboembolic, and wound complications, to name a few.10 The increased risk of mortality has been shown to persist for several years after transfusion.11
The intricate nature of the relationship between anemia, blood loss, transfusions, and patient outcomes needs to be understood.12,13 Perioperative anemia is the major predictor for perioperative transfusion,14 and hence when studying the impact of either anemia or transfusion on the outcomes, the confounding effect of each factor must be considered. Nonetheless, various studies have shown that each factor does remain independently associated with worse outcomes when adjusted for comorbidities.12 This leads to 3 main conclusions: first, that anemia should be screened for, properly diagnosed, and treated; second, that perioperative blood loss should be minimized; and third, that transfusion, as a default treatment, is often not an appropriate management strategy for anemia.15
Here, we discuss strategies to manage anemia in the perisurgical setting, as illustrated by 3 representative patients undergoing cardiac, orthopedic, and oncologic surgeries.
Case 1: anemia in cardiac surgery
A 64-year-old woman with known alcohol dependence presented to her primary care physician with worsening shortness of breath when walking up 2 flights of stairs to her apartment. She had also noticed occasional chest pain on exertion, especially during warmer temperatures. An electrocardiogram in the office was unchanged from baseline 2 years ago, but based on the patient’s history, an outpatient cardiac stress test was scheduled.
Her medical history was remarkable for hypertension, hyperlipidemia, and asthma. Past surgical history included femur fracture after an accident 20 years earlier related to driving under the influence. Her social history was remarkable for consuming 1 to 1.5 bottles of red wine per evening and smoking marijuana 1 to 2 times per month. The patient denied tobacco use, was a vegetarian, was divorced, and was employed. Medications included metoprolol and an albuterol inhaler as needed.
The patient underwent an exercise stress test, which was terminated due to dyspnea and ST depression in anterolateral leads. Coronary angiography showed multivessel coronary artery disease with a normal ejection fraction. A decision was made to perform coronary artery bypass grafting.
Preoperative assessments
The patient’s height was 1.52 m and her weight was 51.7 kg (body mass index [BMI], 21.5). The patient was afebrile with a heart rate (HR) of 72, blood pressure (BP) of 134/83 mmHg, a respiratory rate of 16, and peripheral capillary oxygen saturation of 96%. The patient was awake and alert and in no acute distress. Cardiac examination indicated regular rate and rhythm with S1 and S2. Her chest was clear to auscultation bilaterally; her abdomen was soft and not distended or tender. The patient had mild hepatomegaly and splenomegaly. The extremities were warm with no noticeable edema.
Preoperative laboratory results are provided in Table 1. Presence of anemia, partially reduced iron markers, and elevated liver enzymes prompted further attention. A peripheral blood smear was positive for macrocytes and hypersegmented neutrophils. Serum B12 and folate levels were normal.
An abdominal ultrasound indicated steatohepatitis and an enlarged spleen but there was no evidence of cirrhosis or abdominal ascites. Surveillance esophagogastroduodenoscopy (due to history of alcohol use) was negative for gastric varices.
Alcoholic liver disease can lead to anemia (which can be macrocytic as was the case with this patient). Nutritional deficiencies causing anemia are common among alcoholic liver patients.16 In more severe cases, bleeding from varices can be a major contributor to iron deficiency. This patient was also a known vegetarian, which could lead to decreased oral iron intake.17 Laboratory testing can show a mixed picture because liver disease can affect parameters such as serum iron, ferritin, transferrin saturation (TSAT), and mean corpuscular volume (MCV).16 Reticulocytes are normally larger than red blood cells (RBCs) and markedly elevated reticulocyte counts can also lead to mildly elevated MCV.
The liver plays a major role in iron homeostasis. It is the main source of hepcidin, the master regulator of iron.18 Elevated hepcidin levels (as seen in inflammation) lead to a decrease in serum iron due to its sequestration and decreased gastrointestinal (GI) absorption. The effects of different etiologies of liver disease on hepcidin, iron hemostasis, and anemia are not yet fully understood.19 Hemolysis is also a common cause of anemia in patients with alcoholic liver disease.16 Lastly, alcohol is a known direct toxin in the bone marrow, causing reversible suppression of hematopoiesis and impaired platelet production.20 This patient did not have severe alcoholic liver disease, but the elevated alcohol intake, signs of alcoholic steatohepatitis, and elevated levels of liver enzymes pointed to a likely etiology for this patient’s anemia.
Preoperative management of anemia
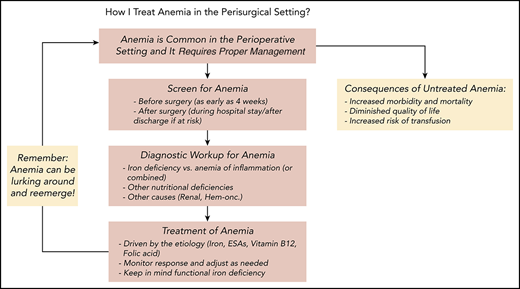
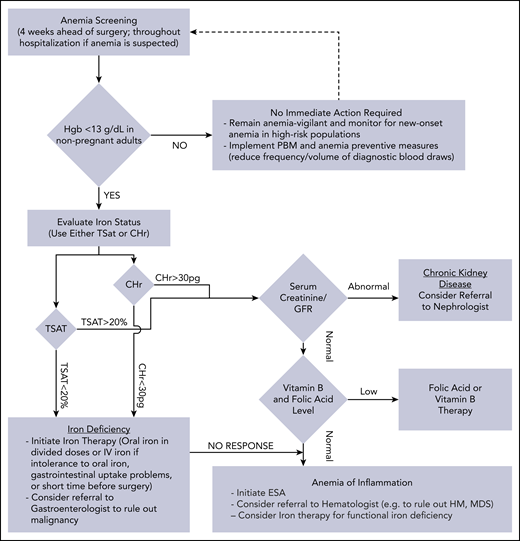
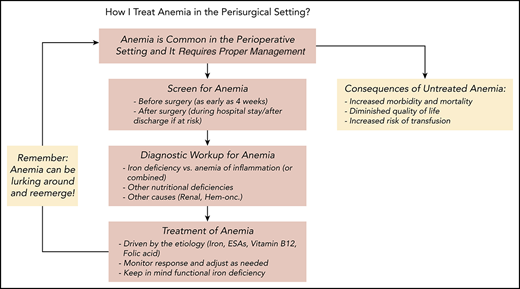
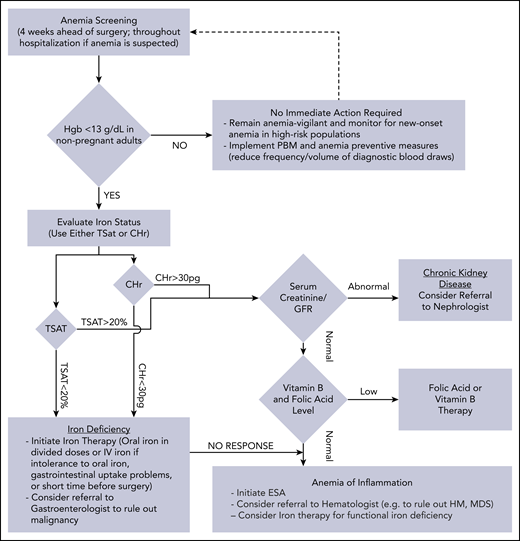
Management of anemia should be directed by the underlying etiology. A proposed algorithm for timely detection, evaluation, and management of anemia in the perioperative setting is provided in Figure 1.21-23
A proposed algorithm for the detection, evaluation, and management of anemia in the perioperative setting. Whenever treatment of anemia of iron deficiency or inflammation is initiated, we routinely add vitamin B12 and folic acid, as well to meet the potential increased demand. The provided algorithm is not intended to be all-inclusive and, depending on the availability, other tests and measurements (eg, soluble transferrin receptor) may be used to assist in making the diagnosis.21-23 CHr, reticulocyte hemoglobin concentration; ESA, erythropoiesis-stimulating agent; GFR, glomerular filtration rate; Hgb, hemoglobin; HM, hematologic malignancy; MDS, myelodysplastic syndrome; PBM, patient blood management; SF, serum ferritin; TSAT, transferrin saturation.
A proposed algorithm for the detection, evaluation, and management of anemia in the perioperative setting. Whenever treatment of anemia of iron deficiency or inflammation is initiated, we routinely add vitamin B12 and folic acid, as well to meet the potential increased demand. The provided algorithm is not intended to be all-inclusive and, depending on the availability, other tests and measurements (eg, soluble transferrin receptor) may be used to assist in making the diagnosis.21-23 CHr, reticulocyte hemoglobin concentration; ESA, erythropoiesis-stimulating agent; GFR, glomerular filtration rate; Hgb, hemoglobin; HM, hematologic malignancy; MDS, myelodysplastic syndrome; PBM, patient blood management; SF, serum ferritin; TSAT, transferrin saturation.
This patient was at increased risk for perioperative blood transfusion due to preexisting anemia and small body size (decreased RBC volume). Hence, she was treated with an erythropoiesis-stimulating agent (ESA; epoetin alfa, 20 000 U subcutaneous daily for 5 days) along with 5 daily parenteral infusions of 200 mg of iron sucrose. We routinely use B12 (1 dose of 1000 µg, intramuscularly) and folic acid (1 mg daily) in anemic patients, which were used for this patient. Following discussion with the patient, she agreed to stop drinking alcohol with the assistance of social work resources and was monitored for withdrawal. Alcohol and substance dependency and abuse are complex issues that often require specialized intervention. Abstention from alcohol will decrease the direct toxic effects on the bone marrow and periods of sobriety can improve nutritional intake. Prior to surgery, the patient’s hemoglobin was 13.1 g/dL.
Intraoperative anemia management
Multiple methods were used for blood conservation including limiting hemodilution associated with large volume expansion, decreased priming volume for cardiopulmonary bypass (CPB) circuit, intraoperative red cell salvage (cell saver), ultrafiltration, and microplegia. A lysine analog (tranexamic acid) was used to decrease fibrinolysis and improve hemostasis. No allogeneic blood transfusion was needed during the surgery.24 Although acute normovolemic hemodilution was not used in this case, it is another blood conservation technique that can be considered for surgeries with potential high blood loss including cardiovascular procedures.25
Postoperative management
The patient had successful revascularization and was transferred to the cardiothoracic intensive care unit. Vasoactive medication support was needed during the first 12 hours postoperatively. Attention was made to maintain body temperature, correct acidosis, and monitor for coagulopathy and excessive postsurgical blood loss (and none was noted).
Laboratory results obtained 24 hours after the surgery (Table 1) were consistent with anemia of acute blood loss and postcardiac bypass–associated thrombocytopenia.26 There was no acute need for ESA but restoration of iron levels remained a priority. For patients unable to tolerate food of high nutritional value, supplemental iron might be appropriate. The Ganzoni formula was used to calculate postoperative iron deficit to aid with parenteral iron supplementation (target hemoglobin level of 13 g/dL with assumed iron stores of 500 mg): total iron dose = [(actual body weight [kg]) × (13 − actual hemoglobin [g/dL])] × 2.4 + iron stores (mg).
Frequency and volume of diagnostic blood draws were kept at minimum during hospital stay and the patient had an eventless recovery and was discharged with recommendation to follow up in outpatient clinic.
Case 2: anemia in orthopedic surgery
A 73-year-old man was scheduled for surgery in 5 days for left total knee arthroplasty due to severe osteoarthritis. His history was notable for hypertension, type II diabetes (last HbA1c 9.1%), chronic kidney disease (glomerular filtration rate [GFR] of 36 mL/min/1.73 m2; Kidney Disease: Improving Global Outcome [KDIGO] stage 3B), and obesity (BMI 35.4). Prior surgeries included appendectomy and right inguinal hernia repair. Current medications included valsartan, verapamil, glipizide, insulin glargine, and ibuprofen for pain control. He had no known medication allergies and no smoking history. He consumed alcohol only a few times a year and denied substance use. He was married and was a retired furniture salesman.
Preoperative assessments
The patient’s height was 1.73 m, and his weight was 105.7 kg. His temperature was 98.7°F, HR was 67, BP was 135/84, respiratory rate was 18, and peripheral capillary oxygen saturation was 98%. He was in no apparent distress, sitting with a cane next to his chair. In the physician’s examination, mild conjunctival pallor was noted. Cardiac examination indicated regular rate and rhythm with S1 and S2; his chest was clear to auscultation bilaterally. The abdomen was obese and soft with bowel sounds present and no palpable mass. A healed surgical scar was evident. Extremities were warm and dry with nonpitting edema. Preoperative blood test results are provided in Table 2.
The patient’s primary care physician had checked for fecal occult blood after seeing initial laboratory results, which was negative. The patient had had a screening colonoscopy 11 months earlier without any abnormal findings. Additional blood tests were performed for anemia evaluation (Table 2). Folic acid and vitamin B12 levels were within normal limits. Coagulation screening tests were normal.
Preoperative management of anemia
Laboratory findings were consistent with anemia of chronic (kidney) disease and iron deficiency.27 Anemia is common in patients with chronic kidney disease (CKD). As the GFR deteriorates, the prevalence of anemia increases, especially among diabetic patients.28 Anemia in patients with moderate to severe CKD is associated with higher mortality and increased risk of end-stage renal disease in male patients not yet on dialysis.29
The anemia of CKD is usually normocytic and normochromic. The main etiology is related to reduced production of endogenous erythropoietin by the kidney and shortened life span of the RBCs.30 Additionally, transcription of hepcidin is induced by inflammatory cytokines present in patients with chronic inflammation, such as CKD.31 The high hepcidin levels often seen in CKD can contribute to functional iron deficiency.32
Iron-deficiency anemia was also present as indicated by reduced serum iron (Table 2). Ferritin can be a misleading marker for iron deficiency as its level can raise in inflammation.33 Direct measurement of the reticulocyte hemoglobin content (mean reticulocyte hemoglobin content [CHr] or reticulocyte hemoglobin equivalent [RET-He]) is a more reliable way of assessing iron stores available for erythropoiesis.34 Low reticulocyte hemoglobin content is a reliable indicator of iron deficiency (true or functional) and iron-restricted erythropoiesis.35
Treatment with ESAs can lead to “functional” iron deficiency (due to increased demand) and therefore, ESA treatment is commonly supplemented with iron. On the other hand, there is also a possibility of functional iron deficiency due to inflammatory response with high levels of hepcidin seen in anemia of inflammation, and in these cases, both ESA (to treat the inflammatory anemia) and iron (because the patient cannot mobilize their endogenous iron stores) are often needed.36 Measurement of soluble transferring receptor is another test that can help in distinguishing anemia of inflammation and iron deficiency.37 Lower levels of iron can be secondary to nonsteroidal anti-inflammatory drugs–induced gastritis and associated blood loss: this patient had increased use of ibuprofen for knee pain. Finally, the patient was receiving valsartan, and angiotensin II receptor blockers are associated with suppression of endogenous erythropoietin production and can contribute to anemia.38
Preoperative anemia treatment plan
Because joint replacement surgeries have a higher risk for blood loss and transfusion,39 it was appropriate to delay the elective procedure and treat the patient to optimize his hemoglobin level.40 Any changes to the surgery schedule should be done with consideration of local logistics and fully discussed with clinical and surgical team. An ESA (epoetin alfa 20 000 U subcutaneously daily for 10 days) was considered.41 In such a situation, the goal is to increase RBC mass and achieve a target hemoglobin level of 13 g/dL prior to surgery. Use of ESAs in shorter durations is less likely to be associated with deep vein thrombosis (DVT) and concurrent administration of DVT prophylaxis is recommended to further reduce the risk.42 The benefits and risks of ESAs were discussed and treatment with ESA along with DVT prophylaxis was initiated.
The use of parenteral iron is preferred when shorter times are available to manage iron deficiency.43 In addition, patients with CKD and decreased GI absorption of iron are less likely to benefit from oral iron therapy.44 Ferumoxytol (510 mg IV push with a second dose 1 week later) is an option to minimize the need for multiple outpatient visits. Combined use of parenteral iron and ESA ensures that adequate iron remain available to meet the need of increased erythropoiesis.45
Physicians were also able to make modifications to the prescribed insulin regimen to improve glucose control. A “prehabilitation” program was prescribed, and the patient was encouraged to continue physical activity (swimming) while being optimized for surgery. The patient returned to the clinic 24 days later for reevaluation prior to scheduling his surgery and new blood tests showed improvements (Table 2).
Intraoperative anemia management
The patient underwent left total knee arthroplasty with 400-mL estimated blood loss. Intraoperative cell recovery was performed and 120 mL of washed shed RBCs was reinfused. ε-aminocaproic acid (EACA), an antifibrinolytic, was administered parenterally during the intraoperative phase to limit fibrinolysis and blood loss. Tranexamic acid is another antifibrinolytic to consider (intravenously or topically) in this setting. Use of topical hemostatic agents (eg, fibrin sealants) is another effective hemostatic measure.46 A surgical tourniquet was not used during the case due to potential for postoperative complications and limitations on rehabilitation.
Postoperative anemia management
Patients should be monitored for postoperative bleeding, and blood cell recovery can be performed if needed. A surgical drain with reinfusion capability (ConstaVac CBCII Blood Conservation System) was inserted in the operating room. Approximately 500 mL of shed blood was reinfused. Postoperative blood test results (Table 2) did not warrant further use of ESA or iron supplementation. Anemia remains a risk for this patient given the comorbidities (namely CKD) and he was advised to undergo subsequent screening and proper management of anemia after discharge.
Case 3: anemia in oncologic surgery
A 67-year-old woman was evaluated for abdominal pain and gastroesophageal reflux symptoms. Esophagogastroduodenoscopy had no significant finding but a subsequent abdominal computed tomography scan identified a mass measuring 2.5 × 3.1 × 2.7 cm in the head of the pancreas. The diagnosis of pancreatic carcinoma was made with a biopsy and the patient was scheduled to undergo pancreatoduodenectomy.
Her medical history was remarkable for hypertension and diet-controlled hyperlipidemia. The patient was taking amlodipine 5 mg, and had no known allergies. The patient used to smoke 1.5 packs of cigarette per day before quitting 36 years earlier. She consumed 2 glasses of wine on weekends but none since diagnosis; she denied substance use. She was married and worked as a preschool teacher. Past surgeries included dilatation and curettage, caesarean section, and right shoulder arthroscopy. Her family history was positive for hypertension and hyperlipidemia. Her mother was alive at the age of 89 years with mild dementia; her father had died at the age of 75 years from congestive heart failure.
Preoperative assessments
The patient’s height was 1.65 m and her weight was 73.9 kg (BMI, 27). She appeared well but anxious. Cardiac examination indicated regular rate and rhythm with S1 and S2. Her chest was clear to auscultation bilaterally; her abdomen was soft, not distended, and nontender to palpation. Bowel sounds were present. No hepatomegaly, splenomegaly, or mass was noted. Extremities were warm with no edema. Rectal examination was normal and a stool guaiac test was negative. Preoperative blood test results are provided in Table 3.
Preoperative management of anemia
Blood test results were consistent with early-stage iron-deficiency anemia. Because the patient was scheduled to undergo a major surgery with high risk for large volume of blood loss and decreased nutritional intake during the postoperative period, it was appropriate to treat her iron-deficiency anemia and replenish iron stores preoperatively. Parenteral iron was chosen given the short time available and considering that the patient’s complaint of abdominal pain was likely to limit tolerance of oral iron.
Intraoperative anemia management
The patient underwent a successful pancreatoduodenectomy (Whipple procedure). She had ∼1.6 L of blood loss. Intraoperative management included cell recovery (salvage) and acute normovolemic hemodilution.
During any surgery with significant blood loss, the shed blood can be collected, washed, and filtered using a cell-saver system to provide a valuable source of autologous blood. Cell-saver use in cancer surgery has been a matter of debate considering the theoretical risk for reintroducing malignant cells into patient’s circulation.47 The alternative option to cell-saver blood is allogeneic blood transfusion, with risks such as immunomodulation, which could negatively affect the patient’s survival.48 Use of a leukocyte-reducing filter during reinfusion was effective in the removal of tumor cells from salvaged blood.49 Studies do not support an increased risk of disease progression or recurrence or decrease survival associated with use of salvaged blood in cancer surgery.50
Postoperative anemia assessment
The patient had an eventless recovery with limited phlebotomy due to her stable clinical status. She was discharged home with a prescription for oral iron with the knowledge that she would continue to need iron supplementation after acute blood loss anemia and with the need for optimization within a few months for additional oncologic treatment. When seen 9 weeks after the surgery, she had reduced appetite and had lost weight, but she was unsure the loss of appetite was related to postsurgical changes or conditions such as depression. She had poor diet, mostly consuming tea, saltines, yogurt, and candy. She indicated that she became fatigued more easily compared with prior to the surgery. She denied shortness of breath or chest pain. She complained of severe GI side effects (abdominal pain, bloating and constipation) following taking the iron pills, which led her to stop taking them shortly after the discharge. She also complained of feeling lightheaded after taking her morning BP pills.
Postoperative computed tomography and positron emission tomography scans showed evidence of locally advanced disease. The patient was scheduled to start neoadjuvant chemotherapy with Folfirnox (folinic acid, 5FU, irinotecan, and oxaliplatin).
This patient had many risk factors for anemia (eg, the underlying malignancy/inflammation, recent high-blood-loss surgery, possible depression, and poor diet) and some physical signs and symptoms of moderate anemia (pallor, increased HR, and lower BP) were present. Her blood tests showed a mixed picture: anemia was normocytic, but the test results were indicative of inflammation (elevated ferritin, low reticulocyte count) as well as iron deficiency (low RET-He). Such a mixed picture of both anemia of inflammation and iron-deficiency anemia is quite common in cancer patients.51,52 Another common reason for anemia in oncologic patients (which was not yet present in this case) is the antineoplastic and myelosuppressive chemoradiotherapy given to treat their cancers.52
As previously discussed, anemia should not be left untreated in any patient but this is particularly important in cancer patients given the additional risk factors. Anemia is associated with reduced survival in many cancer types.53,54 In a case like this, anemia should be addressed as the patient is optimized to start neoadjuvant chemotherapy.
Postoperative anemia management
Addressing underlying etiologies is important for proper management of anemia. In this case, given the poor diet and possible depression, options including support group, nutritional consulting, as well as referral for pharmacologic treatment to address depression and their impact on quality of life and diet were considered. Concern was raised that the patient showed signs of malnutrition (6.8 kg weight loss in 9 weeks), which was further supported by her history of inadequate caloric intake and food of poor nutritional quality. A consultation for nutritional support in the context of both oncologic disease and anemia was requested.
The patient’s low RET-He was indicative of formation of iron-deficient RBCs. As previously discussed, although the ferritin level is normally decreased in iron-deficiency anemia, inflammation (active malignancy and recent surgery in this case) can elevate ferritin levels as an acute phase reactant.33 Inflammation can also result in lower TIBC contrary to the higher levels seen with iron-deficiency anemia. On the other hand, transferrin is a negative acute-phase reactant. Because the patient had not tolerated oral iron supplementation, she was an excellent candidate for parenteral iron infusions. Parenteral administration of iron is also appropriate to shorten the time frame to improve anemia and optimize patient RBC mass to start neoadjuvant chemotherapy.
The inflammatory component of anemia in this patient also needed attention. Concerns related to the use of ESAs in oncologic patients include a possible effect on disease progression and decreased survival.55 The most recent guidelines by the American Society of Clinical Oncology (ASCO) and American Society of Hematology (ASH) largely limit the use of ESAs in cancer patients only to those with chemotherapy-associated anemia undergoing treatment with not-curative intent when hemoglobin is <100 g/dL, while reinstating RBC transfusions as an alternative.56 However, design flaws of some of the earlier studies have come to light and more recent meta-analyses are generally supportive of use of ESAs in this patient population.40,55 As mentioned earlier, leaving patients anemic in fear of possible risks of a treatment such as ESA is not a risk-free alternative as it will expose the patients to other perils: risks of anemia as an adverse risk factor for survival in oncologic patient population, as well as the risks of allogeneic blood transfusion that might be given to them as a temporary relief.12 Suggested protocols for treatment of anemia with hemoglobin levels of 8 to 10 g/dL in this population include epoetin alfa 40 000 U subcutaneous weekly or 10 000 U subcutaneously 3 times per week
In our management of these patients, if the hemoglobin level does not increase by at least 1 g/dL after 4 weeks of therapy, the dose of ESA can be increased to 60 000 U per week or 20 000 U thrice weekly. On the other hand, if hemoglobin level increases >1 g/dL in 2 weeks, the epoetin alfa dose should be reduced by 25%. Lastly, if there is no response after 8 weeks, epoetin alfa administration should be completely stopped.
Studies generally support the safety and efficacy of restrictive transfusion strategies in cancer patients similar to most other patient populations.57,58 Allogeneic blood transfusion should only be reserved as a last resort when there is immediate need to increase RBC mass, with the potential risks and benefits carefully weighed. As previously indicated, oncologic patients who receive blood transfusions are noted to have worse outcomes including increased thrombotic events, in-hospital mortality, and decreased survival.53,54,59 This patient had a hemoglobin value of 8.7 g/dL and she did not show any signs or symptoms of end-organ dysoxia or ischemia and therefore did not have indications to receive a blood transfusion. While the patient was being prepared for chemotherapy, her hemoglobin level increased in response to parenteral iron and ESA within a few weeks and she remained under observation to ensure that she did not become anemic again.
Summary
Anemia is not an innocent bystander and its presence, particularly in the perioperative setting, should never be ignored. The cases presented here cover many aspects of management of anemic patients commonly encountered in clinical practice. As it is becoming increasingly the norm, patients have significant comorbidities that further complicate their anemia.
It has been recommended that patients scheduled for elective surgery undergo anemia screening as early as 4 weeks prior to the scheduled procedure (or even earlier if possible) to allow adequate time for management of anemia if present.21 The value of this early detection strategy can be seen in these 3 cases, as in all, the patients underwent anemia treatment prior to surgery to make sure their RBC mass was optimized ahead of surgery. In the second case, the surgery was postponed to allow enough time for this important step.
Treatment of anemia should be guided by the etiology and a proposed algorithm is provided in Figure 1. It is important to point out that hematinic deficiencies such as iron deficiency in absence of anemia can still independently lead to unfavorable outcomes while increasing the subsequent risk of development of anemia and hence, there is a strong case supporting their treatment even in absence of anemia.60 The anemia management strategy should be tailored according to each patient’s specific needs and conditions. Oral iron supplementation faces limitations in terms of patients’ tolerance and therapeutic response rate, but studies suggest that administration of oral iron as single, low daily dose (40-50 mg/day) or moderate doses on alternate days (80-100 mg) could be at least as effective while being better tolerated than when given at high or divided doses.61,62 In 2 of the cases here, the increased risk and need to optimize hemoglobin level in a shorter time interval led to use of ESAs and parenteral iron concurrently.
Another important aspect of management of the anemic patient in the surgical setting is minimizing blood loss. Several blood conservation strategies are available that can reduce surgical blood loss and they are indispensable tools in preserving an already limited and compromised resource: the patient’s own blood.63,64
Finally, it is important to remember that management of anemia does not end with the surgery. Anemia remains a significant and common problem in the postoperative setting. Continues monitoring of at-risk patients for anemia, continuing with anemia treatments as needed, and adhering to blood management strategies (minimizing iatrogenic blood loss due to unnecessary or wasteful diagnostic blood draws as a common example)65 are some key strategies. The third case presented here is an example of how anemia management can be interrupted once the patient leaves the hospital, placing the patient at a higher risk without proper planning and foresight.
Acknowledgment
The authors express their gratitude to Mazyar Javidroozi for editorial assistance.
Authorship
Contribution: All authors reviewed and approved the final submission.
Conflict-of-interest disclosure: A.S. has received consulting fees and honoraria from Vifor Pharma, Daiichi, and Pharmacosmos. L.T.G. has been a consultant and member of the medical advisory board of American Regent. M.K. declares no competing financial interests.
Correspondence: Lawrence T. Goodnough, Department of Pathology, School of Medicine, Stanford University, 300 Pasteur Dr, Office Suite H-1402, Stanford, CA 94305; e-mail: ltgoodno@stanford.edu.
REFERENCES
Author notes
A.S., M.K., and L.T.G. contributed equally to the manuscript.