Blood groups are defined by membrane proteins that are either single-pass, multi-pass, or glycosylphosphatidylinositol-linked proteins. The antigens defining the blood groups can be either the proteins themselves or the complexes of sugars that decorate these membrane proteins. For antigens that are present at high frequency on red blood cells, transfusion incompatibility problems, due to the absence of undefined blood group antigens, may cause difficulty in finding matching blood. This transfusion complication can only be remedied when the identity of the blood group antigen is discovered. In this issue of Blood, Azouzi et al,1 using a combination of complementary “omic” approaches, reveal the molecular identity of a rare blood group (PEL) that was first reported almost 40 years ago by Daniels et al,2 the high frequency antigen, ABCC4 (an ATP-binding cassette [ABC]-transporter).
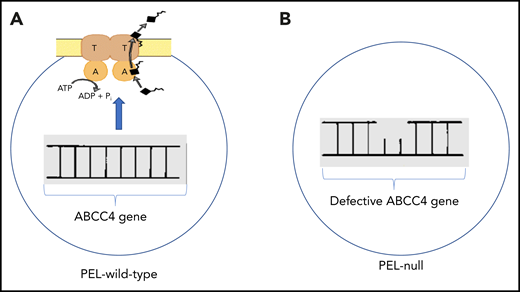
Panel A illustrates an intact ABCC4 gene encoding the plasma membrane ABC transporter. ATP is hydrolyzed providing the energy to remove substrates (black rectangle with irregular line). Panel B illustrates a defect in the ABCC4 gene characteristic of individuals lacking ABCC4 protein, a characteristic of the PEL-null blood group.
Panel A illustrates an intact ABCC4 gene encoding the plasma membrane ABC transporter. ATP is hydrolyzed providing the energy to remove substrates (black rectangle with irregular line). Panel B illustrates a defect in the ABCC4 gene characteristic of individuals lacking ABCC4 protein, a characteristic of the PEL-null blood group.
Several ABC transporters are abundant in red cell membranes, and two have recently defined rare blood groups, Lan and Junior.3,4 ABC transporters harbor conserved functional domains for binding and hydrolyzing the transport energy source, ATP, as well as variable membrane spanning domains for binding substrates. Some ABC transporters reside in intracellular organelles, but Azouzi et al use anti-PEL antisera to confirm that ABCC4 is located at the plasma membrane. There it can export important biological molecules, such as prostaglandin E2 (PGE2) and cyclic adenosine monophosphate (cAMP) (see figure), the latter being a key molecule regulating receptor-mediated platelet aggregation, and the former being a potential modulator of platelet aggregation.5 Interestingly, among ABC transporters where substrate overlap occurs, loss of 1 transporter can lead to a compensatory increase in the expression of the other,6 suggesting this might occur in PEL-null individuals.
Platelets have a key role in maintaining hemostasis by reacting to blood vessel injury. Their adherence to the site of injury activates surface receptors (eg, glycoprotein VI, GPVI, the collagen receptor) to elicit the release of chemicals from platelets (eg, adenosine diphosphate [ADP]) that attract other platelets, which ultimately form a plug of platelets as the primary component of thrombi. ABCC4 was recently implicated as a regulator of platelet activation in 2 different Abcc4 knockout mouse strains.7,8 For PEL-null individuals, ADP-induced aggregation was markedly impaired. A similar finding was observed in certain Abcc4 knockout mice, which is interesting because PGE2 reportedly modulates ADP-induced platelet aggregation.5 However, as this defect only occurred in 1 strain of Abcc4-null mouse, it opens the possibility that compensation in the platelet activation pathway occurs. Nonetheless, both Abcc4 knockout strains display reduced thrombus formation induced either in vivo by vascular injury or in an ex vivo model system.7,8 Given ABCC4 exports cAMP (see figure), it was not surprising that both strains lacking ABCC4 also displayed evidence of activated cAMP/protein kinase A signaling. Platelet activation downstream from collagen’s interaction with its receptor (GPVI) is regulated by cAMP. Surprisingly, for PEL-negative individuals, collagen-induced platelet aggregation was essentially normal. This was unexpected. One speculation to account for this is that constitutive absence of ABCC4 in humans promotes compensatory mechanisms that conceal the impact of ABCC4 absence on collagen-induced aggregation. Because acute chemical inhibition of ABCC4 in a human was shown to attenuate collagen-induced platelet aggregation,7 this seems conceivable. Azouzi et al do show that ABCG2 is upregulated in the plasma membrane of red cells, which supports this idea, and seems plausible given some nucleotide substrates show overlap between ABCC4 and ABCG2.6,9 The authors speculate that this might explain why, at this early stage of characterization of the PEL-null individuals, no discernible defect is detected in red blood cells.
Given that ABCC4 is broadly expressed in multiple hematopoietic lineages, there are important implications for the current findings. As ABCC4 extrudes a variety of cancer chemotherapeutic agents, the authors speculate that ABCC4 membrane expression detected by the anti-PEL antisera might be used to determine if plasma membrane amounts of ABCC4 in patients with hematologic malignancies relate to therapeutic response and/or prognosis. This is an attractive and worthwhile proposal. Another aspect, related to platelets, but not considered, is that high-platelet ABCC4 expression might contribute to platelet hyperreactivity, a property often associated with the greater thrombotic risk and observed in diseases such as atherosclerosis, cancer, and diabetes. Indeed, a recent report suggested an association between high-platelet ABCC4 expression in patients with osteoarthritis and platelet hyperreactivity.10 Altogether, the paper by Azouzi and colleagues not just uncovers the molecular identity of a high-frequency red blood cell antigen but also characterizes the anti-PEL antisera, which will be useful for identifying factors that affect ABCC4 plasma membrane expression.
Conflict-of-interest disclosure: The author declares no competing financial interests.