Background: Acute myeloid leukemia (AML) is the most common acute leukemia in adults with a median age at diagnosis of close to 70 years. (Juliusson G, et al. Blood, 2011;119(17):3890-3899) The majority of patients with AML require allogeneic stem cell transplant (Allo-SCT) in order to achieve cure. European LeukemiaNET (ELN) published risk stratifications for AML patients according to cytogenetics in 2010 and then updated risk categories in 2017 to incorporate molecular abnormalities as well. The ELN adverse risk category predicts for a higher risk of relapse and worse overall survival (OS) compared to favorable and intermediate risk patients. (Döhner H, et al. Blood, 2017;129(4):424-447) Older patients are more likely than younger patients to have adverse risk disease. (Mrozek K, et al. JCO, 2012;30(36):4515-4523). In addition, older patients are typically not considered for myeloablative conditioning (MAC) regimens due to the high risk of non-relapse mortality (NRM). Thus, a significant proportion of AML patients who are candidates for Allo-SCT have adverse risk disease by ELN criteria and are only candidates for RIC Allo-SCT, both of which increase the risk of relapse.
A recent randomized trial in AML and MDS patients noted a trend toward improved OS and statistically significant improvement in relapse free survival (RFS) favoring MAC over RIC (Scott BL, et al. JCO, 2017; 35(11): 1154-1161). Despite this, studies have shown durable remissions and long term disease free survival with RIC Allo-SCT in AML patients with high risk features. (Tauro S, et al. JCO, 2005; 23(36):9387-9393) However, to our knowledge no study has investigated the use of RIC Allo-SCT exclusively in patients who have adverse risk by 2017 ELN criteria. Thus, we reviewed AML patients with adverse risk disease by 2017 ELN criteria at diagnosis who underwent RIC Allo-SCT at our institution.
Methods: We conducted a single center retrospective study of high risk adult AML patients by ELN criteria who underwent RIC Allo-SCT at MUSC from 3/20/2010 to 2/20/2018. Adverse risk disease was defined by the 2017 ELN criteria. Baseline demographic, clinical, laboratory, pathology, and outcomes data were collected by retrospective chart review. Kaplan Meier was utilized for time to event analysis.
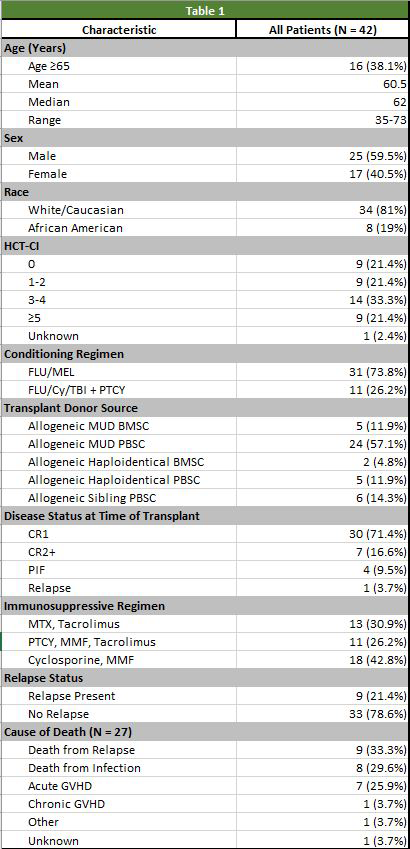
Results: A total of 42 patients with adverse risk AML received RIC Allo-SCT. The median follow up was 16.6 months. Clinical characteristics were collected for each patient and are included in table 1. The median age was 62 with 16 patients (38%) ≥ 65 years of age. Twenty-three patients had a HCT-CI score ≥ 3 (54%) with 9 patients (21%) having a HCT-CI score ≥ 5. Complete remission (CR) was defined as bone marrow blasts < 5% without known molecular evidence of persistent/relapsed AML. CR and CR with incomplete count recovery (CRi) was not able to be defined. Thirty-one patients (73%) were in CR1 at transplant, 7 patients (16%) were in CR2+ defined as CR necessitating ≥ 2 treatment regimens. Four patients (9%) never achieved a CR and were labelled as primary induction failure (PIF), and one patient (3%) received prior MAC Allo-SCT before receiving RIC Allo-SCT and was labeled as Relapse. Four of the five patients with disease status of PIF or relapse were deceased within 13 months of Allo-SCT. The non-relapse mortality (NRM) at 1 year was 26.2%. Relapse-free survival (RFS) at 1, 2, and 3 years was 57.1%, 45.2%, and 35.7% respectively. The overall survival (OS) at 1, 2, and 3 years was 66.7%, 50.0%, and 40.5% respectively. A subset analysis of the patients in CR1 or CR2+ prior to RIC-Allo SCT noted a NRM at 1 year of 27% along with a RFS at 1, 2, and 3 years of 59.5%, 48.7%, and 40.5% respectively. Cause of death included relapse (33%), infection (29%), acute GVHD (25%), and chronic GVHD (3%).
Conclusion: This provides evidence that although adverse risk disease by ELN criteria and reduced intensity conditioning both increase the incidence of relapse in patients with AML, that RIC Allo-SCT for this high risk patient population can provide long term disease free survival. In addition, the favorable outcomes for patients in CR1 and CR2+ prior to RIC-Allo are promising. It would be interesting to investigate a larger multi-center series of patients with adverse risk disease by ELN in CR prior to RIC Allo-SCT. Minimal residual disease by myeloid molecular panel and/or flow in this patient population prior to transplant would be of great interest as well.
Edwards:Genzyme: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.