Background: As the use of lenalidomide (Len) combinations for the management of multiple myeloma (MM) broadens in frontline, Len-refractoriness is a growing issue among the relapsed MM patient (pt) population and raises the need for efficient Len-sparing treatment options. However, current data with Len-sparing combinations is limited. Here we report the results of a real-life cohort of MM pts with at least 1 prior treatment line, who received carfilzomib (CFZ) and dexamethasone (Kd), by Len-refractory (LR) status.
Methods: The prospective cohort study (NCT03091127) recruited adults with relapsed or refractory MM who received ≥1 dose of a CFZ combination regimen in routine clinical practice. In the 10 participating countries across the EU and Israel, 56 mg/m2 twice-weekly was the only approved Kd regimen. Pts were classified as LR if they met at least one of the following 3 criteria to any prior regimen containing Len as defined in the report of the International Myeloma Workshop Consensus published by Rajkumar et al. (Blood 2011 [117] 4691-4695): best response was stable or progressive disease; progression was the reason for stopping any regimen; or/and date of relapse/progression was after the start date and within 60 days after the last dose of Len. Pts were followed until 18 months after CFZ initiation or until 30 days after final administration, whichever was earlier.
Results: Of the 93 pts who received Kd between 14 March 2017 and 22 October 2018 (cut-off date of planned interim analysis), over two-thirds (69%, n=64) were LR at Kd initiation. Median age at CFZ initiation was 70 years for the overall Kd population and LR pts. Pt characteristics, where available, were also similar in terms of ISS stage or ECOG status 0-2 at Kd initiation. However, where reported (18/93 pts), 8 of the 9 pts with a high cytogenetic risk were in the LR subgroup. LR pts had received a median of 3.5 prior lines of therapy compared with 2 for non-LR (NLR) pts. Only 3 LR pts were at first relapse compared with 10 for the NLR pts. In the NLR subgroup, 41% had been exposed to Len. Time since last prior treatment discontinuation was much shorter for LR vs NLR subgroup: 0.8 vs 3.3 months.
Comorbidities were similar regardless of LR status, with over one-third of pts with hypertension and 20% of cardiac disorders (mainly cardiac arrhythmia) at MM diagnosis.
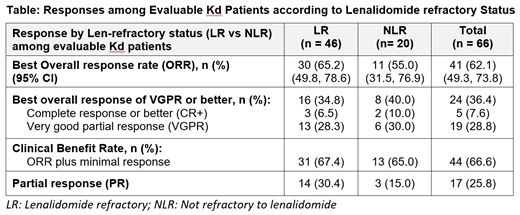
The overall response rate (ORR) among evaluable pts was 65% for LR pts and 55% for NLR pts (Table). Minimal response was reported for 1 LR pt and 2 NLR pts, which resulted in a similar clinical benefit rate (CBR) for LR and NLR pts (67% vs 65%, respectively). Regardless of LR status, at least one-third of Kd pts achieved a very good partial response or better (VGPR+).
The vast majority of pts (92% of LR vs 93% of NLR) planned a twice-weekly Kd schedule. The mean proportion of CFZ label dose received was slightly lower for LR (71%) vs NLR (79%) pts. The Kaplan-Meier estimate of median treatment duration (95% confidence interval) was shorter for the LR vs NLR subgroup: 7.1 months (5.1, 9.6) vs 9.5 months (5.5, 19.0), respectively. At the time of this interim analysis, 39% of LR pts and 52% of NLR pts were still on treatment.
Out of pts who discontinued CFZ, a higher proportion of LR vs NLR pts discontinued mainly due to disease progression/refractoriness (51% vs 29%, respectively). The occurrence of AEs of grade 3 or above (Gr3+) was comparable between LR (38%) and NLR pts (41%) and consisted mostly of infections (15%) and blood disorders (10%). Four out of 5 Gr3+ cardiac events were reported in LR pts, including 2 cases of heart failure leading to CFZ discontinuation. Four of the 5 fatal events occurred in LR pts.
Conclusion: Compared with the ENDEAVOR Kd pivotal trial population, this real-world Kd cohort was older (median 70 vs 64 years), received Kd in later lines (median number: 3 vs 2) and included a higher proportion of LR pts (69% vs 24%). Higher levels of discontinuation due to Gr3+ cardiac and fatal events were observed in the more heavily pre-treated LR pts. Response to treatment was similar irrespective of LR status, and ORR (65%) was similar to ENDEAVOR Len-exposed Kd pts with 2-3 prior lines. Kd benefit-risk profile was favorable in this real-world cohort and appears to be a suitable Len-sparing treatment option.
Leleu:Carsgen: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Oncopeptide: Honoraria; Karyopharm: Honoraria; Amgen: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Merck: Honoraria. Caers:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gamberi:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Heibl:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Honoraria; Pfizer: Honoraria; Mundipharma: Honoraria; AOP Orphan Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Suzan:Amgen (Europe) GmbH: Employment, Equity Ownership. Mohammad:Amgen Ltd.: Employment, Equity Ownership. Wetten:Amgen Ltd.: Employment, Equity Ownership. Terpos:Takeda: Honoraria, Other: Travel Grant, Research Funding; Janssen: Honoraria, Other: Travel Grant, Research Funding; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.