Introduction: Myelofibrosis (MF) is a chronic Philadelphia chromosome-negative myeloproliferative neoplasm characterized by progressive bone marrow (BM) fibrosis, extramedullary hematopoiesis, splenomegaly, constitutional symptoms, and shortened survival. Data pertaining to the clinical characteristics and treatment patterns of patients with low-risk MF are limited; most studies have focused on patients with intermediate- and high-risk MF. The ongoing Myelofibrosis and Essential Thrombocythemia Observational STudy (MOST) was designed to characterize the demographics, disease burden, patient-reported outcomes, and management of patients with MF or essential thrombocythemia (ET) in clinical practices throughout the United States (NCT02953704). This analysis describes demographic and clinical characteristics of patients with low-risk MF enrolled in MOST.
Methods: MOST is an ongoing multicenter, non-interventional, longitudinal, prospective, observational study in patients with MF or ET. Eligible patients with MF were at least 18 years old and had low- or intermediate-1 (INT-1) risk by age alone according to the Dynamic International Prognostic Scoring System (DIPSS). Patients participating in blinded investigational drug trials, having life expectancy ≤6 months, or having other concurrent myeloid malignancies were excluded. Data from patient records were entered into an electronic case report form during usual-care visits over a planned 36-month observation period. Data were analyzed with descriptive statistics.
Results: A total of 232 patients with MF were enrolled between November 29, 2016 and March 29, 2019 at 124 sites. Two-hundred patients with low-risk (n=77) or INT-1 risk by age alone (n=123) MF were included in this analysis (data cut-off date, June 17, 2019); 32 patients were excluded due to incorrect risk categorization (n=27) or unanswered prognostic factors at enrollment (n=5). At enrollment, the median age was 68 (range, 35-88) years, 58% were aged >65 years, 49% were women, and 89% were white. Thirteen patients (7%) had a documented family history of MF, ET, or polycythemia vera. Of 157 patients with manual spleen assessment at enrollment, 55 (35%) had palpable splenomegaly; median spleen length was 7 (range, 1‒22) cm in 35 patients with available measurements.
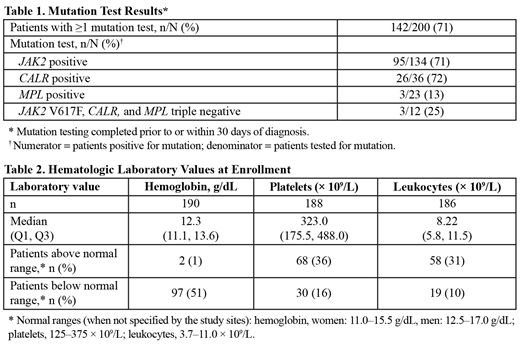
The median time from MF diagnosis to enrollment was 1.7 (range, 0.0-37.7) years; most patients (75%) were diagnosed within 5 years of enrollment. Of patients with available data, 93% (185/200) were reported to have undergone BM biopsy/aspiration and 82% (162/198) had mutation testing (MT) at the time of diagnosis; most patients had received both BM biopsy and MT (151/196 [77%]). Data from MT conducted prior to or within 30 days of diagnosis were available for 142 patients (71%); 134/142 patients (94%) were tested for a JAK2 mutation, of whom 95/134 (71%) were positive (Table 1). At enrollment, approximately half of patients with available data (97/190 [51%]) had hemoglobin below normal range, and approximately one-third had platelets (68/188 [36%]) or leukocytes (58/186 [31%]) above normal range (Table 2).
The most common signs reported at the time of enrollment included lactate dehydrogenase greater than the upper limit of normal (41%), palpable spleen (31%), and leukocytosis (>11 × 109/L; 24%). Across both risk groups, 111 patients (56%) were receiving MF-directed monotherapy at enrollment (low-risk, 43/77 [56%]; INT-1 by age alone, 68/123 [55%]). Low-risk patients received hydroxyurea (HU; 23/43 [54%]), ruxolitinib (15/43 [35%]), interferon (4/43 [9%]), or anagrelide (1/43 [2%]); INT-1 patients received ruxolitinib (30/68 [44%]), HU (28/68 [41%]), interferon (8/68 [12%]), or anagrelide (2/68 [3%]). Five patients (3%) were receiving >1 MF-directed therapy. Less than half of low- (34/77 [44%]) and INT-1- (50/123 [41%]) risk patients were receiving no MF-directed therapy at enrollment.
Conclusion: These real-world data provide insight into the clinical characteristics, diagnosis, and treatment patterns of patients with low- or INT-1 risk (by age alone) MF in the United States. Data from this trial will help characterize the rate at which patients transition from low- or INT-1-risk disease to higher risk categories of disease and how management is affected by disease progression.
Gerds:Sierra Oncology: Research Funding; Imago Biosciences: Research Funding; Incyte: Consultancy, Research Funding; Celgene Corporation: Consultancy, Research Funding; CTI Biopharma: Consultancy, Research Funding; Pfizer: Consultancy; Roche: Research Funding. Lyons:Texas Oncology: Equity Ownership; Amgen: Consultancy; McKesson: Other: Leadership. Colucci:Incyte: Employment, Equity Ownership. Kalafut:Incyte: Employment, Equity Ownership. Paranagama:Incyte: Employment, Equity Ownership. Verstovsek:Promedior: Research Funding; Blueprint Medicines Corp: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Pragmatist: Consultancy; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.