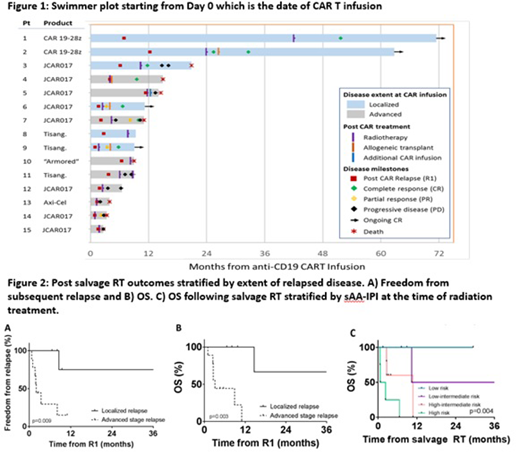
Anti-CD19 chimeric antigen receptor modified T-cells (CAR T) produce remarkable responses for relapsed/refractory diffuse large B-cell lymphoma but over half of patients will relapse. Disease progression post CAR T therapy remains challenging and without standard guidelines. Radiotherapy is potentially an important salvage approach, but limited data exists to guide optimal utilization. We reviewed the first 15 patients treated with salvage radiotherapy (SRT) following CAR T progression. All but two patients had DLBCL; one had blastoid variant mantle cell lymphoma and the other patient was best classified as a CD5+ high grade lymphoma not otherwise specified. Outcomes included post RT response, freedom from subsequent relapse (FFSR) and overall survival (OS). Patients were heterogeneous and heavily-treated with a median of 4 prior lines of pre-CAR T therapy (range 2-8). Median time to first post-CAR T relapse was 82 days and RT was part of the first salvage regimen for 73%. Most received SRT to a site that was previously avid pre-CAR T. Post SRT, there were 6 evaluable patients with limited stage relapse; objective response rate was 100% (n=3 CR and n=3 PR). For advanced-stage relapse, while many had in-field PR, nearly all had concomitant out-of-field progression. Median OS from first post-CAR T relapse was 11 months. Patients with localized vs. advanced stage relapse had significantly improved FFSR (hazard ratio, HR=0.11, p=0.009) and OS (HR 0.10, p=0.003). By second-line age-adjusted IPI (sAA-IPI), 40% were low or low-intermediate risk and lower sAA-IPI risk prognosticated improved OS post SRT (p=0.004). Four patients were bridged to allogeneic transplantation with SRT and at analysis, 3 were alive and NED with 4.9, 7.2 and 36.2 months of post-allogeneic transplant follow-up. The fourth patient was also NED but deceased from transplant-related complications. SRT following CAR T is feasible with powerful and diverse utility including local palliation for transplant-ineligible patients. For lower risk and localized relapses, SRT may be integrated with novel targeted agents, immunotherapies or transplantation to attempt durable remissions.
Palomba:Hemedicus: Other: Immediate Family Member, Speakers Bureau ; Merck & Co Inc.: Other: Immediate Family Member, Consultancy (includes expert testimony); Seres Therapeutics: Other: Immediate Family Member, Equity Ownership and Membership on an entity's Board of Directors or advisory committees; STRAXIMM: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees; Kite Pharmaceuticals: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Noble Insights: Consultancy; Evelo: Other: Immediate family member, Equity Ownership; MSK (IP for Juno and Seres): Other: Immediate Family Member, Patents & Royalties - describe: intellectual property rights . Sauter:Novartis: Consultancy; Sanofi-Genzyme: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; GSK: Consultancy; Spectrum Pharmaceuticals: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy; Celgene: Consultancy; Kite/Gilead: Consultancy. Scordo:Angiocrine Bioscience, Inc.: Consultancy; McKinsey & Company: Consultancy. Batlevi:Juno Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Park:Allogene: Consultancy; Amgen: Consultancy; AstraZeneca: Consultancy; Autolus: Consultancy; GSK: Consultancy; Incyte: Consultancy; Kite Pharma: Consultancy; Novartis: Consultancy; Takeda: Consultancy. Shah:Janssen: Research Funding; Amgen: Research Funding. Giralt:Celgene: Consultancy, Research Funding; Takeda: Consultancy; Sanofi: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Perales:NexImmune: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Medigene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Research Funding; Miltenyi: Research Funding; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sadelain:Juno Therapeutics: Consultancy, Patents & Royalties, Research Funding; Fate Therapeutics: Consultancy, Patents & Royalties; Memorial Sloan Kettering Cancer Center: Employment.
Author notes
Asterisk with author names denotes non-ASH members.