Background
Follicular lymphoma (FL) is the second most common lymphoma accounting for approximately 15% of all non-Hodgkin's lymphoma (NHL). Yttrium-90 ibritumomab tiuxetan [(90)Y-IT; Zevalin] is a radio-immunoconjugate (RIC) which targets CD20. It is approved in relapsed/refractory low-grade NHL and as consolidation after upfront induction chemoimmunotherapy for FL. This study analyzed patients with previously untreated (UFL) or relapsed/refractory low-grade FL (RFL) treated at our institution with (90)Y-IT. It represents the largest reported cohort for therapeutic use of (90)Y-IT in low-grade FL.
Methods
Medical records of patients with low-grade FL (WHO grade 1-2) who received treatment with (90)Y-IT at Mayo Clinic Cancer Center between January 2003 and December 2018 were analyzed. Overall response rate (ORR) and complete response rate (CR) were calculated. Progression-free survival (PFS), time to next therapy (TTNT), and overall survival (OS) were analyzed using the Kaplan-Meier method.
Results
Our cohort consists of 137 patients - 29% (40/137) with UFL and 71% (97/137) with RFL. The median age at diagnosis was 60 years (range, 18-86) with 54% (74/137) males. The median number of previous treatments in RFL patients was 1 (range, 1-5). ECOG performance status at the time of treatment was 0 in 90% and 1 in 10% of patients. 87% (119/137) had stage III/IV disease at the time of (90)Y-IT therapy.
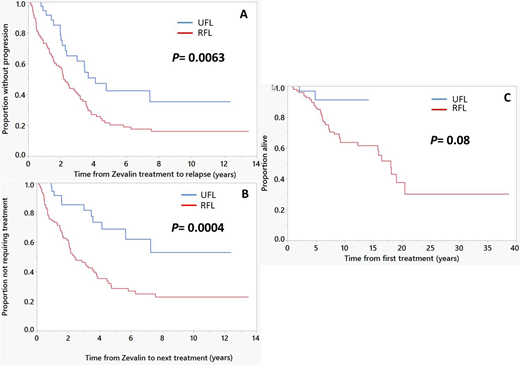
The median follow up from the time of (90)Y-IT therapy was 10.2 years (95% CI; 8.8, 11.6); 69% (95/137) of patients are alive. The ORR was 100% in UFL with 93% (37/40) CR while ORR in UFL was 93% (90/97) with 73% (71/97) CR. 45% (48/108) of the CR patients remain in continuous CR (CCR) with a median follow-up of 7 years (95% CI; 5.2, 9.9). CCR was observed in 55% (22/40) of UFL patients compared to 27% (26/97) of RFL patients. 63% (86/137) of patients had relapsed. More relapses occurred in the RFL group (69/97; 71%) compared to the UFL group (17/40; 43%), (p=0.002). In the entire cohort, the median PFS was 2.5 years (95% CI; 2.1, 3.5) and TTNT was 3.6 years (95% CI; 2.5, 4.7). Median PFS was significantly higher in UFL group compared to RFL group- 4.1 years (95% CI; 2.3, NR) vs 2.2 years (95% CI; 1.6, 3.1), respectively (Figure 1-A). Median TTNT was higher in UFL group compared to RFL group- NR (95% CI; 4.1 years, NR) vs 2.4 years (95% CI; 2, 3.6), respectively (Figure 1-B). Median OS (entire cohort) was 18 years (95% CI; 15.8, NR) with no statistically significant difference between UFL group and RFL group; NR (95% CI; NR, NR) vs 18 years (95% CI; 12.3, 20.5), respectively (Figure 1-C).
Transformation to high grade lymphoma was seen in 19% (18/97) in RFL group compared to 2.5% (1/40) in UFL group, (p=0.005). Median time to transformation was 4.3 years (range, 1-11). More patients developed therapy-related myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) in the RFL group 14% (14/97) compared to 2.5% (1/40) in the UFL group (p= 0.02). Median time to develop MDS/AML was 3.3 years (range, 0-9.7).
Grade ³3 neutropenia was observed in 46% of patients with median time to recovery of 21 days (range, 2-706). Grade ³3 thrombocytopenia was observed in 47% of patients with median time to recovery of 23 days (range, 7-548). Eighteen patients required growth factors support and 14 required transfusions. Non-hematologic AEs included mild to severe fatigue in 35 patients.
Conclusion
Radio-immunoconjugate therapy with (90)Y-IT is an effective single-agent regimen for low-grade FL. The response and survival data in this large real-world cohort is superior to the pivotal trials of this agent conducted nearly 20 years ago. The data in untreated FL is also of interest and provides the rationale for our current randomized phase 2 trial in untreated FL (https://clinicaltrials.gov/show/NCT02320292). Long-term complete remission (>7 years) was seen in 35% of the study population. Based on these promising results, clinical trials combining RIC with novel agents that could potentiate its effects are warranted.
Figure 1
(A) Progression-free survival; comparing time to progression or death after (90)Y-IT treatment between previously untreated patients (UFL) and patients with relapsed/refractory FL (RFL), (B) Time to next therapy; comparing time to starting next line of treatment after (90)Y-IT treatment between UFL and RFL, (C) Overall survival; comparing time to death from all causes after (90)Y-IT treatment between UFL and RFL.
Tun:DTRM Biopharma: Research Funding; TG Therapeutics: Research Funding; BMS: Research Funding; Curis: Research Funding; Celgene: Research Funding; Mundi-pharma: Research Funding.
Yttrium-90 ibritumomab tiuxetan [(90)Y-IT; Zevalin] is a radio-immunoconjugate (RIC) which targets CD20. It is approved in relapsed/refractory low-grade NHL and as consolidation after upfront induction chemoimmunotherapy for FL. We are discussing its use as a frontline therapy in low grade FL.
Author notes
Asterisk with author names denotes non-ASH members.