Background: While mantle cell lymphoma (MCL) initially responds to frontline therapies, this aggressive B-cell malignancy typically relapses or becomes resistant to treatment. Despite high overall response rates to the oral, covalent, first-in-class Bruton's tyrosine kinase (BTK) inhibitor, ibrutinib, most patients with relapsed/refractory MCL ultimately experience disease progression. To address the critical issue of BTK inhibitor resistance, novel therapies must be developed. Moreover, diversity in genetic alterations and the possibility that multiple pathway aberrations may contribute to disease progression and resistance makes overcoming this phenomenon with uniform treatment regimens extremely difficult, indicating that a personalized approach should be developed to overcome therapeutic resistance. In this study, molecular profiling of ibrutinib-resistant MCL patient samples has been conducted to identify targetable dysregulated signaling pathways and gene mutations associated with ibrutinib resistance. Clinical drug candidates targeting these potential pathways and their combinations with ibrutinib were analyzed in vitro to identify novel treatments that may potentially overcome ibrutinib resistance.
Methods: Twenty-three agents targeting multiple pathways associated with ibrutinib resistance were deliberately chosen based on known targetable pathways in MCL to assess via high throughput drug screening. MCL tumor cells were isolated and purified from clinical apheresis and tumor excisional biopsies under established IRB-approved protocols. The same patient primary cells were subjected to gene expression analysis using a nanoString nCounter panel and whole exome sequencing (WES) to identify targetable dysregulated pathways and somatic mutations within each tumor. In vitro cell viability assays of single agents and drug combinations were tested per patient sample using the CellTiter-Glo luminescent assay (Promega), interrogating dysregulated pathways identified in each tumor. Subcutaneous, intravenous, and subrenal injections of the purified patient tumor cells were performed on NSG mice to create corresponding PDX mouse models for validation experiments.
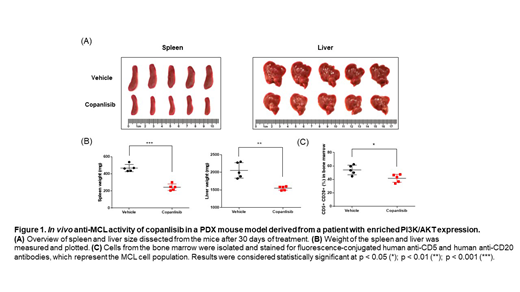
Results: nanoString nCounter analysis identified differentially expressed targetable pathways per patient sample such as BCR signaling, the PI3K/AKT pathway, NOTCH signaling, the cell cycle, and the NF-κB pathway. Correlations were identified between the WES and the nanoString nCounter analysis. For example, PTEN loss was observed in an MCL patient sample with high PI3K/AKT expression, demonstrating the potential underlying mechanism for the observed PI3K/AKT enrichment. Patients were divided into subgroups based on the identified responsive pathways in the in vitro screening. For example, PI3K/AKT pathway inhibitors were shown to be more potent against MCL samples in which the PI3K/AKT pathway was enriched. To further validate this finding, we created an MCL PDX model using a sample with enriched PI3K/AKT expression and successfully recapitulated the splenomegaly and hepatomegaly observed in the MCL patient. The MCL PDX mice were treated with the pan-PI3K inhibitor copanlisib (IP, 10 mg/kg) using a 5 on/2 off dosing schedule, which resulted in significantly reduced spleen (P < 0.001) and liver size (P < 0.01), as well as bone marrow involvement (P < 0.05), compared with the vehicle control (Figure 1).
Conclusions: Molecular matching with in vitro drug screening were utilized to develop a precision medicine platform for MCL to combat therapeutic resistance. This platform can be translated into a clinical setting to directly benefit the MCL patient population through treatment with therapies directly tailored to each patient.
Wang:Pulse Biosciences: Consultancy; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Acerta Pharma: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Dava Oncology: Honoraria; Kite Pharma: Consultancy, Research Funding; Juno Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; MoreHealth: Consultancy, Equity Ownership; BioInvent: Consultancy, Research Funding; Aviara: Research Funding; BeiGene: Research Funding; Loxo Oncology: Research Funding; VelosBio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.