Introduction: While the use of immune checkpoint inhibitors (ICI) is now an established standard of care in the management of many solid tumors, the efficacy of this approach in myeloid neoplasms (MN) is still being explored. Preliminary data regarding efficacy presented to date are modest at best. Since many leukemia physicians lack extensive experience using ICI, and MN patients experience deep thrombocytopenia and bleeding risk precluding lung or colon biopsies to assess for immune-related adverse events (irAEs), MN presents a particularly difficult space for ICI development. Particular concern may center around distinguishing progressive infections (especially fungal) from immune pneumonitis and the appropriateness of steroids in such settings We report a single-center experience on the use of ICI in MN.
Methods: We retrospectively analyzed data from patients (pts) with acute myeloid leukemia (AML) or higher-risk (International Prognostic Scoring System [IPSS] INT-2 or high-risk) myelodysplastic syndromes (MDS) treated with ICI therapy from April 16th, 2015 to June 3rd, 2019. We collected data on patient age, sex, race, Performance Status (ECOG PS), WBC, hemoglobin (Hgb), platelet count, serum metabolic and coagulation parameters, AML disease risk (cytogenetic and molecular) or MDS risk by IPSS score, prior lines of therapy (for refractory/relapsed [R/R] pts), ICI used (classified by targeted immune checkpoint to maintain trial confidentiality) as well as concurrent leukemia-directed therapy, irAEs, transfusion requirements, response to therapy and overall survival (OS).
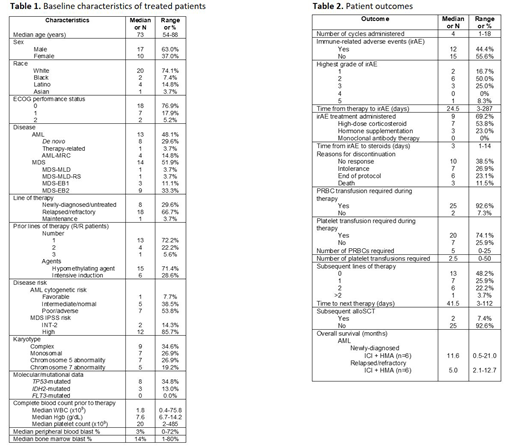
Results: Twenty-seven (13 AML and 14 MDS) pts received ICI in the context of clinical trials. Median age was 73 years, 63% were male, and 95% had an ECOG PS of 0 or 1 (Table 1). Nineteen pts (59%) had R/R disease, of whom 14 previously-received hypomethylating agent (HMA) therapy while 5 received intensive induction therapy. Median number of prior therapies was 1 (range [R], 1-3). Median follow-up was 11.3 months (R, 0.5-46.7). 54% of AML pts had cytogenetically or molecularly-defined poor risk disease; 86% of MDS pts had IPSS high-risk disease. Complex karyotype and TP53 mutation was noted in 35% and 35% of pts, respectively. (Table 1). Fifteen pts (56%) received a PDL1 inhibitor in combination with a HMA, while 4 (19%) pts received either a PD1 inhibitor in combination with a histone deacetylase inhibitor or CTLA4 inhibitor monotherapy. Two pts (7%) received dual ICI with both a PDL1 inhibitor and CTLA4 inhibitor (Table 1). The median number of ICI cycles administered was 4 with the main reason for discontinuation being lack of response (39%). Notably 27% discontinued because of intolerance to therapy. Almost half (n=12, 44%) of pts experienced an irAE; 4 pts had a grade 3 irAE. There were no grade 4 irAEs, but one patient with MDS developed multi-organ grade 3 irAEs and despite appropriate steroid therapy died due to irAEs with necrotizing myocarditis (with multifocal lymphocytic inflammatory infiltrate) and pericarditis confirmed on post-mortem examination. Of pts experiencing an irAE, 69% (including all pts with grade 3 irAEs) received high-dose corticosteroid therapy with a median time to initiation of 3 days (R, 1-14). No biopsies were performed due to bleeding risk in setting of cytopenia. Two of six newly-diagnosed AML pts achieved CR in response to ICI+HMA; one of 2 newly-diagnosed MDS pts developed CR following ICI+HMA. No R/R AML or MDS pts responded. Half of pts proceeded to an additional line of therapy after ICI. Two pts (7%) proceeded to allogeneic stem cell transplant (alloSCT), both receiving anti-CTLA4 monotherapy x 4 cycles with last cycle 62 and 400 days prior to alloSCT, respectively. One pt developed stage 1 acute graft versus host disease (GVHD) of gut with complete response to steroids and another with moderate, classic chronic GVHD without unusual GVHD. No AML pts in this study remain alive. Median OS for newly-diagnosed AML pts treated with ICI + HMA was longer than those with R/R disease (11.6 vs. 5.0 months) at a median follow-up of 11.6 months (Table 2).
Conclusions: Patients with AML and MDS treated with ICI have a significant risk of irAEs requiring provider diligence and possibly quick and life-saving intervention. Larger prospective studies of ICI in AML and MDS patients are needed to better define the role of ICB in management of these cancers.
Podoltsev:Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AI Therapeutics: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Research Funding; Arog Pharmaceuticals: Research Funding; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Research Funding; Astellas Pharma: Research Funding; Daiichi Sankyo: Research Funding; Sunesis Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Pfizer: Research Funding; Astex Pharmaceuticals: Research Funding; Celgene: Other: Grant funding, Research Funding; Genentech: Research Funding; Samus Therapeutics: Research Funding; Kartos Therapeutics: Research Funding. Prebet:Genentech: Consultancy; Boehringer Ingelheim: Research Funding; pfizer: Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; novartis: Honoraria; Boehringer Ingelheim: Research Funding; novartis: Honoraria; Tetraphase: Consultancy; novartis: Honoraria; pfizer: Honoraria; novartis: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; Boehringer Ingelheim: Research Funding; pfizer: Honoraria; Agios: Consultancy, Research Funding; novartis: Honoraria; pfizer: Honoraria; pfizer: Honoraria. Gore:Celgene Corporation: Consultancy, Research Funding. Isufi:Celgene: Consultancy; Novartis: Consultancy; Astra Zeneca: Consultancy. Foss:Seattle Genetics: Consultancy, Other: fees for non-CME/CE services ; miRagen: Consultancy; Mallinckrodt: Consultancy; Spectrum: Other: fees for non-CME/CE services ; Acrotech: Consultancy; Eisai: Consultancy. Huntington:Celgene: Consultancy, Research Funding; Bayer: Consultancy, Honoraria; AbbVie: Consultancy; Pharmacyclics: Honoraria; Genentech: Consultancy; DTRM Biopharm: Research Funding. Neparidze:Eidos Therapeutics: Other: Member of Independent Diagnostic Committee; MMRF/Synteract: Membership on an entity's Board of Directors or advisory committees; Janssen Scientific Affairs, LLC: Research Funding. Zeidan:Daiichi Sankyo: Honoraria; Cardinal Health: Honoraria; Otsuka: Consultancy, Honoraria, Research Funding; Seattle Genetics: Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Medimmune/AstraZeneca: Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Trovagene: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Research Funding; Jazz: Honoraria; Ariad: Honoraria; Agios: Honoraria; Novartis: Honoraria; Astellas: Honoraria; BeyondSpring: Honoraria; Acceleron Pharma: Consultancy, Honoraria, Research Funding; Celgene Corporation: Consultancy, Honoraria, Research Funding.
Immune checkpoint inhibitor therapy is not approved for use in patients with acute myeloid leukemia or myelodysplastic syndrome.
Author notes
Asterisk with author names denotes non-ASH members.