Key Points
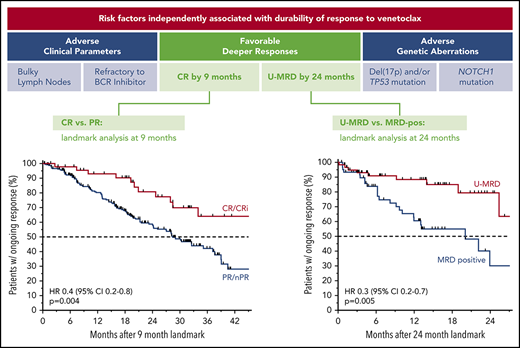
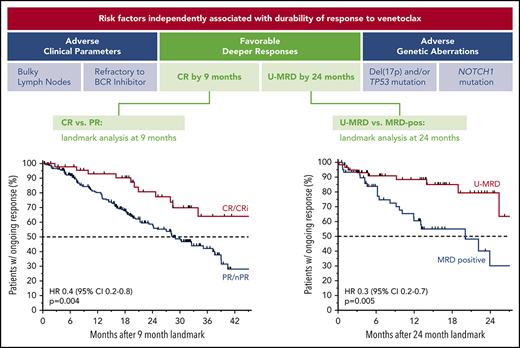
Patients with relapsed CLL achieving complete remission or undetectable MRD on venetoclax treatment have the most durable responses.
Less durable responses are associated with bulky adenopathy, TP53 aberrations, NOTCH1 mutations, and prior refractoriness to BCRis.
Abstract
To define the efficacy of venetoclax with extended follow-up and identify clinical or biological treatment effect modifiers, updated data for previously treated patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) enrolled in 4 early-phase trials were pooled. Rates of response, complete remission (CR/CRi), and undetectable minimal residual disease (U-MRD) were analyzed for all patients (n = 436) and for those patients who were planned to receive 400 mg/day monotherapy (n = 347). Univariate and multiple regression analyses were performed to identify the pretreatment factors associated with response rates and duration of response (DoR). Objective responses were documented in 75% of all patients, including 22% CR/CRi. Overall, 27% and 16% of the patients achieved U-MRD in blood and marrow, respectively. Estimated median progression-free survival (PFS), DoR, and time to progression were 30.2, 38.4, and 36.9 months, respectively. Similar efficacy outcomes were observed within the 400 mg/day monotherapy subset. For those who achieved CR/CRi, the 3-year PFS estimate was 83%. DoR was superior for patients achieving CR/CRi or U-MRD in landmark analyses. In multiple regression analyses, bulky lymphadenopathy (≥5 cm) and refractoriness to B-cell receptor inhibitor (BCRi) therapy were significantly associated with lower CR rate and shorter DoR. Fewer prior therapies were associated with higher CR rate, but not DoR. Chromosome 17p deletion and/or TP53 mutation and NOTCH1 mutation were consistently associated with shorter DoR, but not probability of response. Thus, both pretreatment factors and depth of response correlated with DoR with venetoclax. Patients without bulky lymphadenopathy, BCRi-refractory CLL, or an adverse mutation profile had the most durable benefit.
Introduction
Daily oral administration of the highly selective BCL2 inhibitor, venetoclax, was first approved by the US Food and Drug Administration for patients with previously treated chronic lymphocytic leukemia (CLL) with chromosome 17p deletion [del(17p)] based on the results of phase 1 and phase 2 clinical trials in patients who had received extensive prior treatment.1-3 In vitro studies also demonstrated that response to venetoclax in CLL is independent of p53 function,4 and clinical responses to venetoclax are similar in CLL with or without del(17p).2,3 Subsequently, approvals by the European Medicines Agency5 and regulatory bodies in other regions included use of venetoclax in patients with CLL regardless of 17p status; although these patients may require prior failure of ibrutinib or idelalisib (inhibitors of B-cell receptor [BCRi] signaling). More recently, the venetoclax-rituximab regimen6 has been approved by the US Food and Drug Administration for treatment of patients with previously treated CLL.
Venetoclax is the first in class of a new family of anticancer drugs, the BH3 mimetics; therefore, much of the focus of prior publications has been on the mechanism of action of venetoclax, the rapidity with which it induces responses, and its efficacy and safety in the first few years of use in heavily pretreated patient populations.2-4,7-9 Considering the depth and durability of responses that were observed with venetoclax treatment in initial reports, it is important to evaluate its efficacy in extended follow-up and to identify patient characteristics and treatment outcomes that correlate with long-term efficacy.
Recently, favorable longer-term safety with venetoclax was reported when used continuously for up to 56 months (median duration of exposure, 16 months) in an analysis of pooled data from phase 1 and phase 2 clinical trials.10 Here, we take a similar approach to define the longer-term efficacy of venetoclax through analysis of extended follow-up data of patients treated in 4 early-phase trials. Specifically, we seek to describe how patient and disease characteristics, such as del(17p), clinical parameters, and other genetic abnormalities, correlate with response and durability of benefit with venetoclax-based therapy. We also explore whether achievement of complete remission (CR) or clearance of minimal residual disease (MRD) is associated with duration of response (DoR), and whether biological and prior treatment variables are associated with outcome in patients who achieve such deep responses.
Methods
Study design
Data were pooled from 4 phase 1 or phase 2 clinical trials: NCT01328626 (M12-175, first-in-human dose escalation study),2 NCT01682616 (M13-365, phase 1b combination with rituximab study),11 NCT01889186 [M13-982, phase 2 del(17p) study],3,12 and NCT02141282 (M14-032, phase 2 prior BCRi study).7,8 Individual trial summary descriptions and key eligibility criteria are shown in supplemental Table 1, available on the Blood Web site. Briefly, venetoclax was given orally as daily monotherapy in all trials, with the exception of the phase 1b M13-365 trial, in which patients also received 6 to 9 doses of rituximab over the course of 6 months; venetoclax dosing was 400 mg/day in the 2 phase 2 trials (del(17p) and prior BCRi trials), and ranged between 200 and 1200 mg/day in the phase 1 trials. All trial protocols were designed jointly by AbbVie, Genentech, and the investigators, and were approved by the local institutional review boards. All patients signed informed consent for their respective trial, and the original studies were conducted in accordance with the International Conference on Harmonization guidelines and the principles set forth by the Declaration of Helsinki. The primary results for each trial, all with shorter duration of follow-up than included here, have been reported previously.2,3,7,8,11,12
Patient population
Patients with relapsed or refractory CLL/small lymphocytic lymphoma (SLL) received venetoclax once daily until disease progression, unacceptable toxicity, or in the phase 1b trial with rituximab, achievement of CR or clearance of MRD to an undetectable level (U-MRD). There was no limitation on the number of prior therapies in the 3 monotherapy trials, although M13-365 had a limit of 3 prior myelosuppressive therapies. All entrants required treatment according to 2008 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines.13 Patients in the first 2 cohorts of the first-in-human trial2 were excluded because of inadequate venetoclax exposure, as were the 5 previously untreated patients enrolled in the del(17p) trial.12 For the present study, patients were considered in 2 cohorts for the primary analyses: a cohort in which all patients planned to receive a minimum of 200 mg/day and a maximum of 1200 mg/day of venetoclax after the initial dose ramp up, and a cohort in which patients planned for treatment with 400 mg/day of venetoclax as monotherapy. In a secondary analysis, a cohort of patients treated with venetoclax monotherapy, but without prior BCRi therapy, was also examined.
End points and outcomes
Response and disease progression were evaluated according to the 2008 iwCLL criteria; definitions of relapse and refractory were also based on these criteria.13 CR, CR with incomplete bone marrow recovery (CRi), partial remission (PR), and nodular PR (nPR) were considered as responses to treatment. Response was assessed by investigators and recorded on a continuous basis. Duration of response (DoR) was defined as the time from first documentation of iwCLL response to disease progression or recurrence in the primary analyses. In specified secondary analyses, duration of response was measured from either the time of best response or a specific landmark time. Time to progression was measured from first dose of venetoclax to disease progression. Progression-free survival (PFS) was measured from the first dose of venetoclax to disease progression or death. In time-to-event measurements, patients were censored at the date of last assessment for those without an event. All disease progression events were included in measurements, regardless of whether this occurred on study drug or after discontinuation. Overall survival (OS) was measured from the first dose of venetoclax to the date of death. For patients with continued survival, data were censored on the date of last study visit or the last known date alive, whichever was later. MRD was assessed by sensitive multicolor flow cytometry in peripheral blood or bone marrow aspirates. Clearance of MRD to U-MRD was defined as a result of less than 1 leukemia cell in 104 nucleated cells in an assay with sensitivity of at least 10−4, at any point after treatment initiation. Where no leukemic cells were detected but the assay sensitivity did not reach 10−4, the assessment was considered not evaluable. Sequencing of TP53, NOTCH1, and SF3B1 genes for mutations was performed centrally. For TP53 mutations, targeted next-generation sequencing was performed encompassing all TP53 exons using the TruSeq Custom Amplicon assay (Illumina, San Diego, CA). Variants were called using Illumina’s Somatic Variant Caller and ERIC recommendations applied for reporting (mutation listed in IARC TP53 database and variant allele frequency >10%).14 Mutations in other genes had a minimum of 3% variant allele frequency, were predicted to be deleterious in at least 4 functional prediction algorithms, and have been previously reported in the catalog of somatic mutations in cancer (COSMIC v71). Fluorescence in situ hybridization (FISH) was assessed in local clinical laboratories, as were data on IGHV mutation state, each by standard methodology. FISH data were analyzed categorically for each locus in isolation. Refractoriness to prior fludarabine therapy was defined as failure to achieve at least a PR, or progression within 6 months of therapy, whereas refractoriness to BCRi was defined as failure to respond or progression while receiving the BCRi. Patients who ceased receiving the BCRi because of intolerance and who subsequently had disease progression were not considered BCRi refractory.
Statistical analyses
Patient-level data from the studies were integrated for analyses. All data are reported as intent to treat unless otherwise specified. In addition to descriptive statistics for efficacy outcomes, exploratory analyses were conducted using Kaplan-Meier estimates for time-to-event efficacy end points: PFS, DoR, and OS. Odds ratios (ORs), hazard ratios (HRs) and associated 95% confidence intervals (CIs) were calculated using univariate, multiple logistical and Cox proportional-hazards regressions; ORs were generated from logistical regressions, whereas HRs were generated via Cox proportional-hazards regressions. Where data for individual FISH or gene mutations were missing, the patient was categorized as having no abnormality or no mutation. Sensitivity analyses were also performed, restricting analyses to a given subset of patients who had complete data on each genetic variable. In univariate analyses, P < .05 was considered to indicate significance. Variables with potential prognostic value and that were collected in a sufficient proportion of patients across all studies were included as covariates in multiple regressions. Stepwise regression was applied in multiple analyses to assess the contributions of covariates’ effects for variable selection. In addition to the final selected covariates, effects of response at the 9-month landmark, U-MRD status in the peripheral blood at 24 months, and combination of rituximab with venetoclax were added individually, using stepwise selection, as applicable, to assess their adjusted effects in the final model. In landmark analyses, patients were classified according to the outcome of their most recent clinical or MRD assessment at, or immediately before, the specified time; for example, patients classified as having CR at 9 months were those with CR at or before 9 months and without progression at 9 months.
Results
Patient characteristics
Across the 4 trials, a total of 445 patients with relapsed or refractory CLL/SLL were enrolled; this study provides analysis of the 436 patients planned to receive a minimum of 200 mg and a maximum of 1200 mg/day after the initial dose ramp up. Of these patients, 387 received venetoclax monotherapy and 49 patients also received rituximab. In total, 347 patients were planned to receive the approved standard dose of 400 mg/day as monotherapy. The demographic and clinical features of all patients (n = 436) and those treated with 400 mg/day of venetoclax as monotherapy (n = 347) are summarized in Table 1. Patients had received a median of 3 prior therapies (range, 1-15 prior therapies). All patients in the NCT02141282 trial had prior failure of ibrutinib or idelalisib (n = 107 and 48, respectively); relatively few patients in the other 3 trials had prior exposure to BCRi therapy (n = 28). The majority of patients had 1 or more characteristics associated with poor prognosis [eg, del(17p) or del(11q), bulky adenopathy ≥5 cm, fludarabine- or BCRi-refractory disease] at study entry. The median follow-up is 35.5 months (range, 0.0-69.1 months) for all patients, and 28.8 months (range, 0.03-64.5 months) for those treated with 400 mg/day venetoclax monotherapy; the median (range) duration of venetoclax therapy for each group is 18.8 (0-68.9) months and 16.6 (0-61.5) months, respectively. For currently active patients, median duration of venetoclax treatment is 32.1 (range, 7.9-68.9) months (supplemental Table 2).
Key outcomes
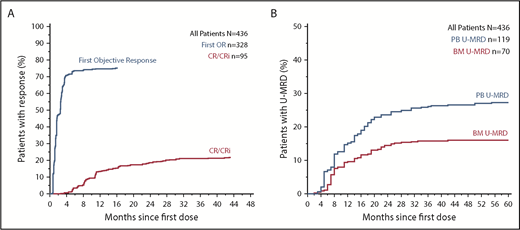
Objective responses were documented in 75.2% (95% CI, 70.9%-79.2%) of all patients; 22.0% (95% CI, 18.2%-26.2%) achieved CR/CRi (supplemental Table 3). For patients intended for treatment with 400 mg/day venetoclax monotherapy, the overall response rate was 73.5% (95% CI, 68.5%-78.1%), with a complete remission rate of 15.9% (95% CI, 12.2%-20.1%). Median time to first response for all patients was 1.6 months (range, 0.5-15.7 months), and most responses were first manifest within 3 months of treatment (Figure 1A). Greater variability was observed in time to CR/CRi (median, 9.3 months; range, 2.6-48.8 months), with 89% of CR/CRi responses achieved by 2 years. Across all patients, 27.3% (95% CI, 23.2%-31.7%) and 16.1% (95% CI, 12.7%-19.8%) achieved U-MRD in the peripheral blood and marrow, respectively, predominantly within 2 years from therapy initiation (Figure 1B). Within the subset of patients treated with 400 mg/day venetoclax monotherapy, 27.1% (95% CI, 22.5%-32.1%) and 10.7% (95% CI, 7.6%-14.4%) achieved U-MRD in blood and marrow, respectively (supplemental Table 3).
Time to response and MRD clearance in all patients receiving venetoclax. (A) Inverted Kaplan-Meier plot showing the cumulative percentage of patients with objective response and CR/CRi as a function of time. (B) Plot showing the cumulative percentage of patients who have documented clearance of minimal residual disease negativity (U-MRD) in peripheral blood (PB) and bone marrow (BM) over time. Analyses in A and B are intent to treat.
Time to response and MRD clearance in all patients receiving venetoclax. (A) Inverted Kaplan-Meier plot showing the cumulative percentage of patients with objective response and CR/CRi as a function of time. (B) Plot showing the cumulative percentage of patients who have documented clearance of minimal residual disease negativity (U-MRD) in peripheral blood (PB) and bone marrow (BM) over time. Analyses in A and B are intent to treat.
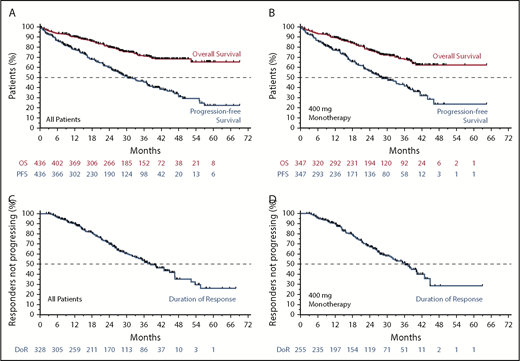
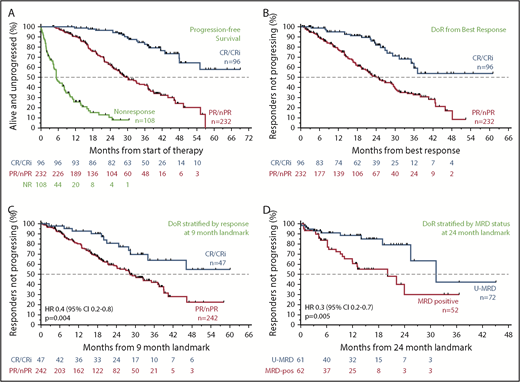
The estimated median PFS was 30.2 (95% CI, 27.2-36.9) months for all patients (Figure 2A; blue) and 28.2 (95% CI, 24.7-34.1) months for 400 mg/day monotherapy (Figure 2B; blue). The estimated median DoR and time to progression for all patients was 38.4 months (95% CI, 33.2-44.9 months) and 36.9 months (95% CI, 30.2-41.5 months), respectively (Figure 2C; supplemental Figure 1A). For those receiving 400 mg/day monotherapy, the median DoR and time to progression were 36.2 months (95% CI, 31.0-40.3 months) and 33.2 months (95% CI, 27.5 - 39.2 months), respectively (Figure 2D; supplemental Figure 1B). Median OS has not been reached; the 3-year survival estimate was 71.3% (95% CI, 66.0-75.9) for all patients and 68.0% (95% CI, 61.6-73.6) for those intended to receive 400 mg/day monotherapy (Figure 2A-B; orange).
Survival and durability of benefit on venetoclax. (A) Kaplan-Meier plot showing the OS and PFS rates of all patients over time; number of patients at risk at each point is shown below the graph. (B) OS and PFS rates of patients who received 400 mg/day of venetoclax monotherapy over time; patients at risk at each point are shown below the graph. (C-D) Duration of response for all patients and for those patients who received 400 mg/day of monotherapy, respectively.
Survival and durability of benefit on venetoclax. (A) Kaplan-Meier plot showing the OS and PFS rates of all patients over time; number of patients at risk at each point is shown below the graph. (B) OS and PFS rates of patients who received 400 mg/day of venetoclax monotherapy over time; patients at risk at each point are shown below the graph. (C-D) Duration of response for all patients and for those patients who received 400 mg/day of monotherapy, respectively.
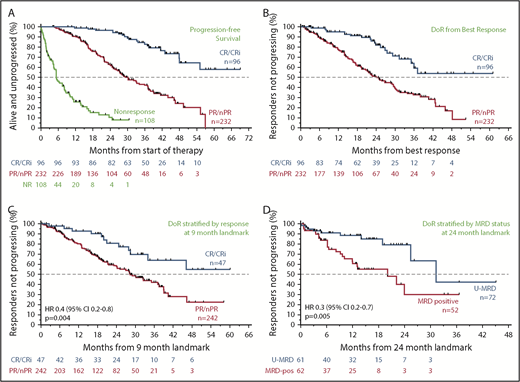
Relationship between the depth of response and durability of response
Across all patients, those who failed to achieve a response to venetoclax had a median PFS of 5.4 months (95% CI, 4.4-8.0 months); in contrast, responders had a median PFS of 39.2 months (95% CI, 35.9-46.5 months). The median PFS for patients who achieved CR/CRi was not yet reached; the 3-year estimate of freedom from progression or death was 83% (95% CI, 72%-90%; Figure 3A). Similar patterns of outcome were observed for patients treated with 400 mg/day monotherapy (supplemental Figure 2A). To address the question of whether a deeper response is associated with more durable benefit, the DoR from the time of best response for patients who achieved CR/CRi was first compared with those patients whose best response was PR. The median DoR for patients who achieved PR/nPR was 24.2 months; for those who achieved CR/CRi, the median DoR has not yet been reached (Figure 3B). Next, to determine the relationship between depth of response and durability of venetoclax benefit independent of the guarantee-time bias inherent in such comparisons, landmark analyses were performed among all responders at the 9-month timepoint, which approximated the median time to CR. The HR for subsequent loss of response if CR was achieved at that timepoint rather than PR was 0.4 (95% CI, 0.2-0.8; P = .004) for all patients (Figure 3C), and 0.2 (95% CI, 0.1-0.8; P = .02) for those treated with 400 mg/day monotherapy (supplemental Figure 2B), indicating a direct relationship between depth and duration of response. This nexus was similarly evident when PFS was analyzed (supplemental Figure 2C-D). Furthermore, clearance of MRD in the peripheral blood was also associated with lower risk for relapse when similar landmark-based analyses were performed for responders at the 24-month point (HR, 0.3; 95% CI, 0.2-0.7; P = .005; Figure 3D).
Progression and durability of response rates over time, according to response depth. (A) Kaplan-Meier plot showing the PFS rates of all patients over time, stratified by best objective response. (B) DoR for all patients, starting from the day of best response, stratified by best overall response. (C) Duration of response for all patients, stratified by response at the 9-month landmark. (D) Duration of response for all patients with available data, stratified by MRD status (in peripheral blood) at the 24-month landmark. Patients at risk at each point are shown beneath each graph. MRD positive, detectable MRD; nPR, nodular PR.
Progression and durability of response rates over time, according to response depth. (A) Kaplan-Meier plot showing the PFS rates of all patients over time, stratified by best objective response. (B) DoR for all patients, starting from the day of best response, stratified by best overall response. (C) Duration of response for all patients, stratified by response at the 9-month landmark. (D) Duration of response for all patients with available data, stratified by MRD status (in peripheral blood) at the 24-month landmark. Patients at risk at each point are shown beneath each graph. MRD positive, detectable MRD; nPR, nodular PR.
Univariate and multiple regression analyses to determine clinical and biological factors that influence response rate
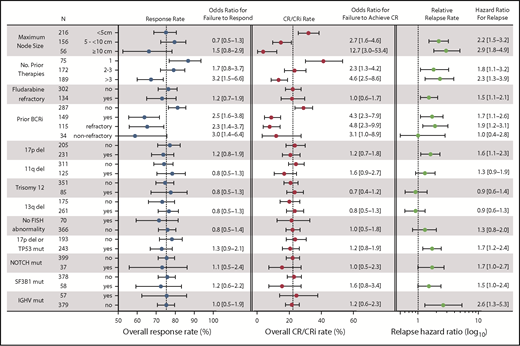
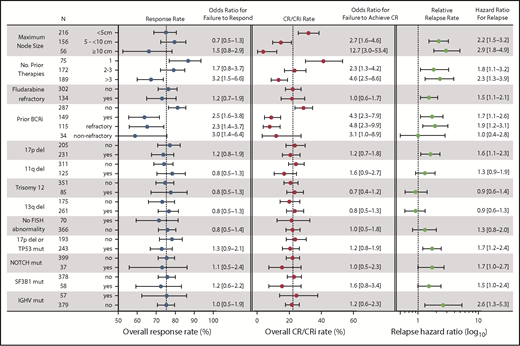
Statistical power derived from the large dataset in this study allowed us to investigate associations between clinical and biological characteristics that have been previously identified as prognostic factors or response modifiers for chemoimmunotherapy and other targeted agents. Receipt of more than 3 prior therapies and prior BCRi therapy were each significantly associated with lower likelihood of achieving any response or CR in the entire population (Figure 4), whereas having received more than 1 prior therapy was associated with a lower likelihood of achieving CR. The presence of bulky adenopathy (ie, lymph nodes ≥5 cm, and especially ≥10 cm in diameter) also was identified as a significant variable negatively associated with the probability of achieving CR; this was not the case for achieving any response (Figure 4). Age; sex; refractoriness to fludarabine, del(17p), del(11q), trisomy 12, del(13q); IGHV mutation state; presence of any TP53 abnormality; or mutations in NOTCH1 or SF3B1 were not correlated with likelihood of achieving a response or CR. The same pattern of univariate results was found for the subpopulation of patients treated with 400 mg/day monotherapy (data not shown), except that del(13q) was associated with a modestly increased likelihood of achieving CR and SF3B1 mutation with reduced chance of achieving CR in that population (OR for failure to respond, 0.4 [95% CI, 0.2-0.9] for del(13q) and 4.6 [95% CI, 1.1-19.5] for SF3B1). In multiple logistical regression analyses, a greater number of prior therapies (particularly > 3), and previous exposure to BCRi retained statistically significant associations with lower rates of response in models with the least unexplained variance (Table 2). For CR, these same factors, as well as larger node size, were significant, with lymphadenopathy larger than 10 cm in diameter being associated with the highest likelihood of failing to achieve CR. In addition, the presence of a TP53 abnormality appeared to marginally associate with reduced chance of achieving response in the population treated with 400 mg/day monotherapy. Conversely, del(13q) remained significantly associated with a higher chance of achieving CR with 400 mg/day monotherapy. Sensitivity analyses that restricted the analyzed data to only those patients with complete genetic data for all loci were also performed; these analyses confirmed the dominant association between prior BCRi exposure and response, and between prior BCRi exposure and lymph node bulk with CR (Table 2; supplemental Table 4).
Summary of univariate analyses of pretreatment factors for association with response outcomes. This figure shows the overall response rates, CR/CRi rates, and relapse rates segregated according to different pretreatment variables, along with the associated ORs and HRs for those values in univariate analyses. (Left) Overall response rate (includes patients with CR, CRi, PR, or nPR); the corresponding OR represents the likelihood for failure to respond compared with the first listed category for each variable. (Middle) Complete response rate; the OR represents the likelihood for failure to achieve CR/CRi. (Right) The relative relapse rate; the HR for loss of response, which is also represented numerically to the right of the graph. The N column represents the number of patients with data for each category. Dotted vertical lines for response rate and CR/CRi rate represent the overall rates observed for all patients. For the relative relapse rate, the vertical dotted line represents a HR of 1.0 (no hazard) associated with the first listed category for each variable). ORs and HRs are shown with 95% CIs for each column. Variables tested but found to be nonsignificant on univariate analyses and not shown in the figure were age (≥70, <70 years) and sex. For these analyses, where data on a cytogenetic abnormality or mutation in TP53, NOTCH1, SF3B1, or IGHV was missing, the patient was included in the no category. del, deletion; mut, mutated.
Summary of univariate analyses of pretreatment factors for association with response outcomes. This figure shows the overall response rates, CR/CRi rates, and relapse rates segregated according to different pretreatment variables, along with the associated ORs and HRs for those values in univariate analyses. (Left) Overall response rate (includes patients with CR, CRi, PR, or nPR); the corresponding OR represents the likelihood for failure to respond compared with the first listed category for each variable. (Middle) Complete response rate; the OR represents the likelihood for failure to achieve CR/CRi. (Right) The relative relapse rate; the HR for loss of response, which is also represented numerically to the right of the graph. The N column represents the number of patients with data for each category. Dotted vertical lines for response rate and CR/CRi rate represent the overall rates observed for all patients. For the relative relapse rate, the vertical dotted line represents a HR of 1.0 (no hazard) associated with the first listed category for each variable). ORs and HRs are shown with 95% CIs for each column. Variables tested but found to be nonsignificant on univariate analyses and not shown in the figure were age (≥70, <70 years) and sex. For these analyses, where data on a cytogenetic abnormality or mutation in TP53, NOTCH1, SF3B1, or IGHV was missing, the patient was included in the no category. del, deletion; mut, mutated.
To investigate whether the dominant negative association of prior BCRi exposure with responsiveness to venetoclax could have masked the effect of other clinical and biological factors, we also analyzed a secondary subpopulation of patients who were naive to BCRi therapy and who received venetoclax 400 mg/day monotherapy (n = 201; patient characteristics described in supplemental Table 5). No additional factors were identified as statistically significantly associated with likelihood for response in univariate analysis (data not shown).
Univariate and multiple regression analyses to determine clinical and biological factors that influence durability of response
As with the analyses focused on response rates, prior exposure to BCRi therapy, higher number of prior lines of therapy, and bulky lymphadenopathy were again associated with less durable responses in univariate analysis (Figure 4, right hand panel). The effect of prior exposure to BCRi was predominantly confined to those patients whose disease was refractory to the BCRi. Additional significant factors were del(17p), mutation of TP53 or NOTCH1, and unmutated IGHV status. In multiple regression analysis, the presence of del(17p)/TP53 mutation and NOTCH1 mutation, as well as IGHV status, lymph node size, and fludarabine-refractoriness and/or BCRi-refractoriness, remained significant for the whole population, but number of lines of prior therapy did not (Table 3, left column). For the population of patients who received 400 mg/day of monotherapy, only BCRi-refractoriness and lymph node size remained significant in multiple regression analysis. In sensitivity analysis restricted to patients with complete informative genetic data, SF3B1 mutation was also independently associated with shorter DoR.
Concomitant rituximab and venetoclax efficacy
The phase 1b M13-365 trial,11 in which patients received 6 to 9 doses of rituximab in addition to venetoclax, reported higher CR rates (51%) and durability of response (estimated 82% PFS at 24 months) than that reported for the monotherapy trials. In the present univariate analysis including the total population, comparison of the CR rate (OR for failure to achieve CR, 0.2; 95% CI, 0.1-0.4) and DoR (HR, 0.5; 95% CI, 0.3-0.8) indicated higher rates of CR and longer DoR with the venetoclax plus rituximab combination. Multiple regression analyses were performed, first including those variables already identified as significant and subsequently adding monotherapy or combination therapy as a variable, to account for the potentially confounding effects of a different distribution of pretreatment factors among the varying trial populations (Table 2). The addition of rituximab was significantly associated with a higher CR rate (OR for failure to achieve CR, 0.4; 95% CI, 0.2-0.8), but not overall response (OR, 0.8; 95% CI, 0.3-1.8). A significant association between addition of rituximab and the durability of response was not confirmed, although a trend to longer DoR (HR for relapse, 0.8; 95% CI, 0.4-1.5) was observed (Table 3).
Both depth of response and pretreatment variables significantly influence response duration
To delineate whether the factors identified above are still impactful on the durability of benefit once depth of treatment response is known (ie, a patient has achieved CR or U-MRD), we incorporated either partial vs complete remission at the 9-month landmark or detectable vs undetectable MRD (in peripheral blood) at the 24-month landmark into the multiple regression analysis in stepwise fashion (Table 3; middle and right columns). These analyses demonstrated that both CR at 9 months and U-MRD at 24 months were independently associated with reduced risk for relapse, whereas all pretreatment parameters (except fludarabine refractoriness when MRD at 24 months was included) remained significant in the multiple regression analysis when all patients were considered.
Discussion
In this pooled analysis of 4 clinical trials, high response rates (75%) and CR rates (22%) were seen with venetoclax in a population of predominantly heavily pretreated patients with multiply relapsed CLL. The consolidated results also emphasize the capability of venetoclax to greatly cytoreduce leukemia burden, with documented clearance of MRD in the peripheral blood in 27% and in the marrow in 16% of patients. Multiple regression analyses identified the significant factors associated with reduced likelihood of achieving a response to be bulky lymphadenopathy (especially >10 cm maximum dimension), previous BCRi therapy, and greater number of prior lines of therapy, particularly more than 3. Consistent with previous publications,2,4 the presence of del(17p) or a TP53 mutation did not correlate with the likelihood of response and only marginally with failure to achieve CR. The only genetic factor associated with more favorable response rates was the presence of del(13q). Patients with del(13q) CLL had a higher likelihood of response and achieving a CR with venetoclax monotherapy.
The estimated median PFS for all patients included in this multitrial analysis of longer-term follow-up data were 30.2 months, and the median DoR was 38.4 months. Durable benefit was clearly associated with the achievement of response, with patients reaching CR having the most favorable outcomes, including in landmark analysis to control for guarantee-time bias. The 3-year PFS estimate for patients achieving CR was 83%. Similarly, where there was clearance of MRD in the blood or marrow documented among responders, the duration of response was significantly longer than when MRD was detected. These findings provide strong evidence of an association between depth of response and durability of benefit with this targeted agent.
Several pretreatment factors were identified that correlated with shorter duration of response, and these can be considered in 3 categories: bulky disease, refractoriness to either fludarabine or a BCRi, and adverse mutation profile (ie, TP53 loss or mutation, NOTCH1 mutation or IGHV unmutated status or potentially SF3B1 mutation). All factors, except IGHV unmutated status, retained independent association in a Cox multiple regression model that included depth of response to treatment, reinforcing that these are each important biological markers of disease with a propensity to progress with ongoing BCL2 inhibition. When similar analyses were restricted to patients who received the standard 400 mg/day monotherapy regimen, patient numbers, and consequently the power to resolve statistically significant associations, were reduced. Nevertheless, lymph node size, disease refractoriness to BCRi, and TP53 loss or mutation were associated with shorter DoR, irrespective of depth of response by iwCLL criteria. A factor that this study could not investigate was karyotypic complexity15 because pretrial testing of karyotype was neither required nor routinely performed. An earlier and smaller study of BCRi-naive patients with relapsed or refractory CLL/SLL treated with venetoclax had indicated that complex karyotype and refractoriness to fludarabine were significantly associated with early progression while TP53 aberrations were not.16 Given that most recurrent CLL with complex karyotype has either TP53 mutation or del(17p), it is possible that these 2 variables largely co-associate in the populations of patients entered on these clinical trials. Because many of the patients included in these early-phase trials had aggressive disease and had been heavily pretreated with chemotherapy, some caution may be required before extrapolating these findings to patients who are treatment naive or have previously received only 1 prior line of therapy.
The strengths of this study are its inclusion of a large number of patients (n = 436) and its relatively mature follow-up of median 35.5 months overall, and 32.1 months in patients still receiving venetoclax. Despite this being the largest study of outcomes for patients treated with venetoclax to date, it has less statistical power within a subgroup of patients treated with 400 mg/day monotherapy. It also has incomplete observational data for some genetic variables and MRD measurements. A lack of power also means that conclusions regarding the effect of the addition of rituximab to venetoclax are provisional. In multiple regression analyses, addition of rituximab was associated with higher CR rate by more than 2-fold compared with venetoclax monotherapy, but no statistically significant correlation with duration of response was observed with current follow-up. Whether the addition of rituximab influences durability of benefit remains an unanswered question. Simple indirect comparisons between single-group monotherapy trials and trials of venetoclax-rituximab combinations suggest superiority for combination treatment; however, those comparisons are confounded because of imbalances in patient populations with respect to several key pretreatment factors identified in the current study, including previous BCRi failure, and TP53 aberrations.6,11 Future comparisons will be able to better address these issues once data from the recently published randomized trial of venetoclax-rituximab versus bendamustine-rituximab are more mature,6 and should include adjustment or matching for the key pretreatment prognostic factors and response modifiers identified herein.
Venetoclax monotherapy achieves deep responses (CR and/or clearance of MRD) in a significant proportion of patients, and patients achieving a CR or U-MRD have excellent durability of response. However, there is clear unmet need for the majority of patients whose disease does not respond as completely. The argument for combination therapy to address this gap is compelling, especially given the first results of the randomized trial reporting the superiority of venetoclax-rituximab over bendamustine-rituximab in patients with relapsed/refractory CLL6 and the preliminary data for venetoclax-obinutuzumab,17 bendamustine-venetoclax-obinutuzumab,18 and venetoclax-ibrutinib-based regimens19 reporting high CR rates. The outcome of ongoing combination trials will determine whether such venetoclax combinations overcome the challenges posed by CLL/SLL with the unfavorable biological and clinical characteristics identified here.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The online version of this article contains a data supplement.
Acknowledgments
Medical writing and assistance was provided by Ryan J. Bourgo, from AbbVie. AbbVie and the authors would like to express their gratitude to all the patients who participated in the included studies and their families. They would also like to thank all of the participating study investigators and coordinators. This manuscript contains information on the investigational use of venetoclax (formerly ABT-199). Venetoclax is being developed in collaboration between AbbVie and Genentech.
AbbVie and Genentech provided financial support for the studies (NCT01328626, NCT01889186, NCT01682616, and NCT02141282) and participated in the design, study conduct, analysis and interpretation of data, as well as the writing, review, and approval of the publication. Investigators also received support from the Australian NHMRC (1079560, 1113577) and Leukemia and Lymphoma Society (A.W.R.), DFG (SFB1074 projects B1 and B2; S.S.), and EU (TRANSCAN FIRECLL; S.S.). Financial support was also received from AbbVie, Inc. and Genentech Inc.
Authorship
Contribution: A.W.R., S.Y.K., and J.F.S provided conception and design; A.W.R., S.M., T.J.K., S.E.C., M.S.D., B.E., M.H., J.C.B., B.C., S.Y.K., S.S., W.G.W., and J.F.S. provided provision, collection, and assembly of data; all authors had access to the data and provided data analysis and interpretation; A.W.R. wrote the first draft of the article; all authors contributed to writing and revision of the manuscript; and all authors provided final approval of manuscript.
Conflict-of-interest disclosure: A.W.R. received research funding from AbbVie and Janssen; is an employee of the Walter and Eliza Hall Institute of Medical Research, which receives milestone and royalty payments related to venetoclax (employees of Walter and Eliza Hall Institute also may be eligible for financial benefits related to these payments); and receives a financial benefit as a result of previous research related to venetoclax. S.M. received research funding from AbbVie, Pharmacyclics, Novartis, Gilead, Acerta, and Incyte; is an advisory board member for AbbVie, AstraZeneca, Genentech, Gilead, Janssen, Pharmacyclics; and provides lecturing for Genentech, Pharmacyclics, and Janssen. T.J.K. is a consultant and received research funding from AbbVie. S.E.C. received research funding from AbbVie, Gilead, and Pharmacyclics. M.S.D. is a consultant and/or advisory board member for AbbVie, Genentech, Roche, Pharmacyclics, Janssen, Gilead, Verastem, TG Therapeutics, Acerta, Astra-Zeneca, Sunesis, and MEI Pharma and received institutional research funding from Genentech, Pharmacyclics, TG Therapeutics, Surface Oncology, and Bristol-Myers Squibb. B.E. received research funding, honoraria (consultant or advisory board member), and travel support from AbbVie, Celgene, Gilead, Janssen, Mundipharma, Novartis, and Roche. M.H. received research funding from GlaxoSmithKline, Mundipharma, Janssen, Celgene, Gilead, AbbVie, and Roche, and is a consultant and advisory board member for AbbVie, Roche, and Genentech. J.C.B. received clinical trial support from Pharmacyclics and Acerta and is an unpaid consultant for Genentech, AbbVie, Acerta, Pharmacyclics, and the Leukemia and Lymphoma Society LLC. K.H. is an employee of Roche and may own Roche stock or options. L.Z., B.C., J.N., J.P., S.Y.K., and M.V. are employees of AbbVie and may hold stock or stock options. S.S. received research funding, honoraria (consultant or advisory board member), and travel support from AbbVie, Amgen, Boehringer-Ingelheim, Celgene, Genentech, Genzyme, Gilead, GSK, Janssen, Mundipharma, Novartis, Pharmacyclics, Hoffmann La-Roche, and Sanofi. W.G.W. received research funding from AbbVie and Genentech. J.F.S. received research funding from AbbVie and Genentech and is a consultant and advisory board member to Roche and Genentech.
Correspondence: Andrew W. Roberts, Clinical Haematology, Royal Melbourne Hospital and Peter MacCallum Cancer Centre, 305 Grattan St, Melbourne, VIC 3000, Australia; e-mail: roberts@wehi.edu.au.