Key Points
Use of ECP prolongs survival and disease control in patients with SS /e-MF.
ECP should be commenced as early as possible in the treatment paradigm for MF/SS, ideally at treatment lines 1 to 3.
Abstract
Extracorporeal photopheresis (ECP) has demonstrated therapeutic benefit in patients with Sézary syndrome (SS) and erythrodermic mycosis fungoides (e-MF). To examine the efficacy of ECP in the modern era of novel therapies, we conducted a retrospective analysis of 65 patients with a diagnosis of SS or e-MF with blood involvement who were treated with ECP at our institute. Overall survival (OS), time to next treatment (TTNT), and skin response rate (RR) were used as the study end points to determine patient outcome. The median follow-up from diagnosis was 48 months (range 1-225 months), with a median predicted OS of 120 months. The majority (88%) of patients commenced ECP at treatment lines 1 to 3, either as a monotherapy or in conjunction with other systemic agents. The use of ECP monotherapy resulted in a significantly longer median TTNT when compared with interferon-α (P = .0067), histone deacetylase inhibitors (P = .0003), novel immunotherapy agents (P = .028), low-dose methotrexate (P < .0001), and chemotherapy (P < .0001). In particular, early commencement of ECP at treatment lines 1 to 3 yielded a TTNT of 47 months. The results of our study support the utilization of ECP for SS/e-MF, and we recommend that ECP should be considered as early as possible in the treatment paradigm for these patients.
Introduction
The existing body of literature for extracorporeal photopheresis (ECP) in the treatment of Sézary syndrome (SS) and erythrodermic mycosis fungoides (e-MF) with leukemic involvement has led to its incorporation into consensus treatment guidelines.1-6 However, previous reports suggest that the long-term prognosis for such patients is poor, with 5-year overall survival (OS) rates of only 27% to 44%.2,7-9
The classic ECP treatment schedule outlined by Edelson et al used in most treatment centers involves ECP on 2 consecutive days once every 4 weeks, for at least 6 months.10 In this paper, we explore the outcome of patients at our institution treated early in their treatment course with a novel 1-day regimen of ECP.
Methods
Study design
This is a retrospective analysis of the Cutaneous Lymphoma Database at Peter MacCallum Cancer Centre with a cutoff date of 16 January 2018. Patients included for analysis were treated between 1997 (when ECP was first introduced at our institution) and January 2018. Data collection and analysis were approved by the Human Research Ethics Committee at our institution and undertaken in accordance with the principles of the Declaration of Helsinki.
Only systemic therapies were analyzed, including ECP, interferon-α (IFN-α), histone deacetylase inhibitors (HDACi: vorinostat, panobinostat, romidepsin), monoclonal antibodies or antibody-drug conjugates or fusion toxins (alemtuzumab, mogamulizumab, pembrolizumab, brentuximab vedotin, denileukin diftitox), bexarotene; IV/oral chemotherapy; low-dose methotrexate; and autologous or allogeneic stem cell transplant. Psoralen and ultraviolet A and total skin electron therapy were also considered systemic therapies based on current treatment guidelines for cutaneous T-cell lymphoma.11 ECP was assessed as either a monotherapy or a combination therapy where administered concurrently or in overlap with other systemic agents. Systemic corticosteroids were excluded due to their indication as a treatment of acute “flares” rather than long-term disease control, as per Hughes et al.12 We enumerated individual treatment lines and divided them into early-line (commenced at lines 1 to 3) or late-line therapy (commenced at line 4 or later).
Data analysis
Primary outcomes were OS (date of diagnosis of mycosis fungoides (MF)/SS to the date of death from any cause or cutoff date), time on treatment (date of commencement of a systemic therapy to the date of cessation due to disease progression or remission, treatment intolerance, or death), time to next treatment (TTNT: date of commencement of 1 systemic agent to date of commencement of the next line of systemic therapy), and skin response rate (RR).
Use of a novel ECP regimen in our cohort
The UVAR-XTS collection device was used at Peter MacCallum Cancer Centre from March 1997 and was replaced by the Therakos CELLEX Photopheresis System in October 2009. A novel treatment protocol for ECP is used at our center, as previously published, consisting of 1 day of treatment per week for 6 weeks, then every 2 weeks for 12 weeks, and then monthly thereafter.13 This differs from the traditional protocol found in European Organization of Research and Treatment of Cancer11 and International Society for Cutaneous Lymphomas11 guidelines, which recommends 2 consecutive treatment days.
Results and discussion
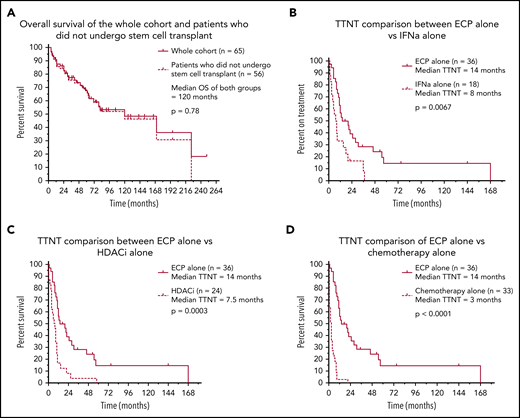
Sixty-five eligible patients were identified, and clinical characteristics are detailed in Table 1. The median time to commencement of ECP was 3 months (range 0-200), and the median follow-up of all living patients was 48 months (range 1-191). A median of 4 (range 1-19) various systemic treatments was administered per patient, with a total of 274 individual treatment episodes. ECP was the most common first-line therapy, with 35 (53.8%) patients receiving ECP at line 1. Of these, 15 were treated with ECP alone, and 20 received ECP in conjunction with another systemic agent. A key finding of this analysis was a median predicted OS of 120 months for patients with SS/e-MF receiving treatment in the modern era. Moreover, this median predicted OS was unchanged when censoring for patients who underwent transplant (autologous stem cell transplant: n = 3; allogeneic stem cell transplant: n = 6) (Figure 1A). To our knowledge, this is the longest reported survival outcome in one of the largest cohorts of its kind to date, exceeding the OS of 39 to 65 months reported in previous studies.14-20 These results may reflect the early commencement of ECP in our cohort, although there were too few patients treated at later lines to permit a formal comparison. At the same time, it is also possible that prolonged OS reflects the focus on immune modulation for the management of SS rather than the use of systemic chemotherapy, which is generally ineffective.
Survival and treatment outcomes in 65 patients with Sézary syndrome who were treated with ECP. Kaplan-Meier curves demonstrating survival and treatment outcomes in our patient cohort. (A) Of the 65 patients who underwent ECP, there was no significant difference in predicted OS between the whole cohort and those who did not receive stem cell transplant (P = .78). Those who used ECP alone demonstrated significantly better TTNT outcomes compared with IFN-α alone (P = .0067) (B), HDACi alone (P = .0003) (C), and chemotherapy alone (P < .0001) (D).
Survival and treatment outcomes in 65 patients with Sézary syndrome who were treated with ECP. Kaplan-Meier curves demonstrating survival and treatment outcomes in our patient cohort. (A) Of the 65 patients who underwent ECP, there was no significant difference in predicted OS between the whole cohort and those who did not receive stem cell transplant (P = .78). Those who used ECP alone demonstrated significantly better TTNT outcomes compared with IFN-α alone (P = .0067) (B), HDACi alone (P = .0003) (C), and chemotherapy alone (P < .0001) (D).
We believe OS and TTNT are key outcomes for evaluating the efficacy of therapy in SS/e-MF. Past studies have generally focused on skin response as the primary measure of treatment efficacy, with previous reports of skin RR ranging from 31% to 85% (complete remission of 0% to 62%).21-24 In comparison, we report a skin response of 69% in our cohort, with a complete remission (skin, blood, node, and viscera) rate of 6%; 26% of patients experienced a stable skin response.
The median time on ECP was 17 months (range 0.5-159), with 57/65 (87.7%) commencing ECP as early-line treatment. Patients receiving ECP as a monotherapy at lines 1 to 3 (n = 20; 30.8%) demonstrated an impressive median time on treatment of 42 months. This extended time on treatment reflects a lengthy period of disease control and confirms the excellent tolerability of ECP and its suitability for long-term use. Furthermore, no grade 3/4 adverse events related to the administration of ECP were reported. With respect to the TTNT outcome measure, patients who used ECP at lines 1 to 3 had a median TTNT of 12 months compared with those who received ECP at later treatment lines (7 months; P = .07). This TTNT is similar to that reported previously.25 Having observed a superior outcome with the early-line use of ECP (time on treatment, TTNT), it raises the question as to why? Is it due to the prior treatments received, and/or the natural course of disease progression over time? Unfortunately, our study was unable to address that question. First, there was an unequal distribution across the lines of therapy with the majority of patients receiving ECP as lines 1 to 3 (n = 57) with only 8 patients receiving it beyond line 3. Moreover, within that early use group, many of our patients were referred from interstate, or even internationally, and received prior therapy as a bridging measure while awaiting planned treatment with ECP; unlike previous studies of chemotherapy or biological agents, the decision to switch to ECP as a second or third line was not necessarily due to inactivity of the prior therapy or refractory disease. Further prospective studies may be able to more clearly characterize natural disease biology vs the disease-modifying effects of ECP.
Beyond line of therapy, we looked for, but were unable to isolate, biological predictors of outcome. For example, with respect to blood stage (B0 vs B2), there was no difference in survival (P = .41) or TTNT (P = .20). Nonetheless, this does support the use of ECP in patients with a range of blood involvement. Similarly, we examined the outcome of patients with large cell transformation (LCT). Because only 3 patients had LCT at the time of commencement of ECP (TTNT = 1 month, 7 months, and 25 months), we were unable to demonstrate any difference in outcome according to LCT status.
The median TTNT when ECP was used alone was 14 months, which was significantly greater than all other systemic therapies: IFN-α (8 months; P = .0067), HDACi (7.5 months; P = .0003), antibody/antibody-drug conjugates/fusion toxins/bexarotene therapy (n = 20; 6.5 months; P = .028), chemotherapy (3 months; P < .0001), and low-dose methotrexate (n = 35; 2.5 months; P < .0001) (Figure 1B-D). We recognize that the above comparisons may be subject to potential bias as to when in the disease course each therapy was administered. However, we note that the TTNT of those treated with ECP in first-line therapy, either as monotherapy or in combination (n = 35), was 12 months, in comparison with a TTNT of only 3.5 months in patients receiving other non-ECP therapies in first line. Taken together, these data strongly suggest that ECP is one of the most effective treatments of patients with SS, even when compared with novel or more targeted therapies. These findings also reaffirm the limited benefit of systemic chemotherapy as a first-line treatment of advanced cutaneous T-cell lymphoma, with a TTNT of only 3 months.
As described above, our institutional protocol allowed the use of ECP with other therapies, although the selection of when and what to use as a treatment partner was at clinician discretion. Patients who received ECP in combination with another systemic agent (n = 37; 69.2%) at treatment lines 1 to 3 had significantly shorter median time on treatment compared with those who received ECP alone (15 months vs 42.5 months; P = .044). Because it is hard to reconcile why combination therapy should result in an inferior outcome, we postulate that patients who were prescribed a combination of ECP and another systemic agent were clinically considered to have more aggressive or rapidly progressive disease at the outset. Indeed, when we examined the outcomes of specific combination therapies, we found that no combination therapy demonstrated a clear superiority to ECP alone. This raises the question of whether ECP combination therapy does in fact offer a therapeutic benefit over ECP monotherapy and warrants further prospective evaluation.
In summary, this analysis demonstrates the efficacy of our novel ECP regimen as an early therapy for patients with SS/e-MF. It achieves prolonged disease control compared with other systemic agents, reflected as prolonged survival, particularly when commenced as a frontline therapy. Whether there is a synergistic effect between ECP and other systemic agents remains unclear and warrants further prospective evaluation.
For original data, please contact the corresponding author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Monash University Medical School for their support.
C.G. was a Bachelor of Medicine and Bachelor of Surgery student within the department in 2018 to 2019.
Authorship
Contribution: C.G. collected and analyzed the data, performed statistical analysis, and wrote the manuscript; H.M.P. designed the project and wrote the manuscript; C.M. and C.v.d.W. provided guidance and assisted in writing the manuscript; R.T. assisted with data collection and analysis and maintained the database; and B.A.C., S.J.H., M.S.G., S.L., C.K., and O.B. contributed to intellectual content and provided feedback on the manuscript.
Conflict-of-interest disclosure: H.M.P. has received research grants and honorarium from Celgene Corporation, Novartis, Merck, and Takeda. S.J.H. has received research grants and honorarium from Celgene Corporation, Novartis, Merck, and Takeda. C.M. has received consultant fees from Merck and Takeda. The remaining authors declare no competing financial interests.
Correspondence: H. Miles Prince, Department of Clinical Haematology, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, Locked Bag 1, A’Beckett St, Melbourne, VIC 8006, Australia; e-mail: miles.prince@petermac.org.