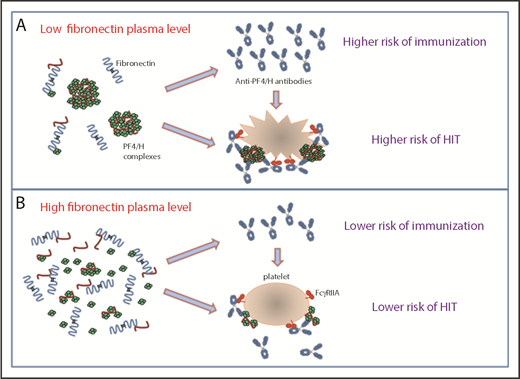
In this issue of Blood, Krauel et al demonstrate that fibronectin, an abundant plasma protein synthesized by the liver, modulates the formation of platelet factor 4 (PF4)/heparin complexes that may influence the risk of heparin-induced thrombocytopenia (HIT).1
The data reported by Krauel et al show that fibronectin could decrease the formation and the size of platelet factor 4/heparin complexes (PF4/H), the risk of immunization to PF4 of heparin-treated patients, and platelet activation induced by HIT antibodies (B). Therefore, low-plasma fibronectin levels could be a risk factor for higher levels of antibodies to PF4/heparin complexes and for HIT (A).
The data reported by Krauel et al show that fibronectin could decrease the formation and the size of platelet factor 4/heparin complexes (PF4/H), the risk of immunization to PF4 of heparin-treated patients, and platelet activation induced by HIT antibodies (B). Therefore, low-plasma fibronectin levels could be a risk factor for higher levels of antibodies to PF4/heparin complexes and for HIT (A).
HIT is a severe complication of treatment with heparin, which is due to immunoglobulin G (IgG) antibodies binding to PF4/heparin complexes and activating platelets after crosslinking FcγRIIA receptors. Remarkably, many patients who synthesize antibodies to PF4/heparin do not develop HIT. In addition, platelets from healthy donors show a wide variability in response to HIT antibodies, particularly when functional assays are performed with platelet-rich plasma. These observations suggest that plasma proteins have a critical influence on the ability of IgG antibodies to PF4/heparin to activate platelets in the presence of heparin. It was recently demonstrated that normal IgG, especially the IgG2 subclass, differentially inhibit HIT antibody-dependent platelet activation based on the FcγRIIA H131R polymorphism, this explaining the higher risk of thrombosis in patients homozygous for the 131R allele.2
In their study, Krauel et al identified 1 plasma protein that interferes with PF4/heparin complexes, HIT IgG antibody-platelet interactions, and subsequent activation. They found that fibronectin, and to a lesser degree vitronectin, inhibited PF4 and PF4/heparin complex binding to platelets. However, only fibronectin strongly inhibited HIT IgG antibody binding to immobilized PF4/heparin complexes. This latter effect was mainly related to a disruption of PF4/heparin complexes (see figure) that probably explains the inhibitory action of fibronectin on HIT antibody-induced platelet activation as demonstrated by the heparin-induced platelet activation test. The precise molecular interactions explaining how PF4/heparin complexes are disrupted by fibronectin are not yet defined.
Fibronectin is an adhesive protein composed of a dimer glycoprotein and is present in 2 different forms, either as an insoluble component of the extracellular matrix or as a soluble and relatively abundant plasma protein. It is involved in a large number of physiological processes, including blood coagulation, the uptake and clearance of blood-borne particles and bacteria by phagocytic cells, wound healing and tissue regeneration, neovascularization, and embryonic development.3 At least 3 heparin-binding sites have been identified on fibronectin, and the 1 present in the C-term part of the molecule (heparin II) is considered the most important. However, according to the data obtained by Krauel et al, all 3 domains appeared necessary for an optimal inhibition of PF4 binding to platelets. However, fibronectin might also directly bind PF4. Interestingly, both proteins are present together in human platelet granules.4
The size and stability of large PF4/heparin complexes on cell surfaces are highly critical for HIT antibody binding, intracellular signal transduction through Fcγ receptors, and subsequent multicellular activation.5 Krauel et al analyzed fibronectin and anti-PF4/heparin IgG levels of 89 patients who had been divided into 4 groups by the results obtained in PF4-specific enzyme-linked immunosorbent assay (ELISA) and serotonin release assay (SRA), a functional test performed with washed platelets. They found that fibronectin levels were significantly lower in the 17 patients having definite HIT than in those (n = 18) without HIT (despite all had developed platelet-activating antibodies as demonstrated in SRA). Therefore, they suggested that low fibronectin levels could increase the risk of HIT in immunized patients by favoring the pathogenicity of anti-PF4/heparin, particularly in specific clinical conditions such as surgery or major trauma.
Interestingly, fibronectin plasma levels are relatively low in the days following cardiac surgery with cardiopulmonary bypass,6 a condition associated with increased circulating PF4 levels and a very high risk of HIT in patients receiving unfractionated heparin. However, this original observation deserves to be confirmed because the samples analyzed by Krauel et al were limited in number. In addition, plasma fibronectin levels are also lower in newborns and children, but they are considered at lower risk for HIT than adults.7
Finally, Krauel et al also proposed that fibronectin could regulate the immune response to heparin because plasma levels of this protein were higher in the 30 patients treated by heparin but who did not synthesize PF4-specific antibodies (negative ELISA). Moreover, data obtained in ELISA and SRA in the 89 patients studied suggested that low fibronectin levels might increase the risk of immunization to PF4/heparin complexes and the occurrence of HIT when IgG antibodies are present (see figure). However, the mechanisms involved in this apparent influence of fibronectin on the HIT immune response remain to be identified. It has been recently demonstrated that PF4/heparin complexes activate complement in plasma and bind to B cells, and this process depends on the size and the charge of immune complexes.8 These 2 variables also affect immunogenicity in murine studies.9 In addition, heparin plays a role in enhancing antigen uptake and activation of the cellular immune response to PF4-containing complexes,10 and the impact of fibronectin on these processes should therefore be investigated.
Conflict-of-interest disclosure: The authors declare no competing financial interests.