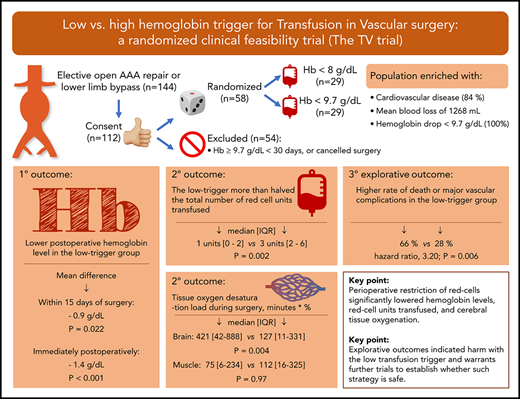
Key Points
Perioperative restriction of red cells significantly lowered hemoglobin levels, red cell units transfused, and cerebral tissue oxygenation.
Explorative outcomes indicated harm with the low transfusion trigger and warrants further trials to establish whether such strategy is safe.
Abstract
Current guidelines advocate to limit red blood cell (RBC) transfusion during surgery, but the feasibility and safety of such a strategy remain unclear, as the majority of evidence is based on postoperatively stable patients. We assessed the effects of a protocol aiming to restrict RBC transfusion throughout hospitalization for vascular surgery. Fifty-eight patients scheduled for lower limb bypass or open abdominal aortic aneurysm repair were randomly assigned, on hemoglobin drop below 9.7 g/dL, to either a low-trigger (hemoglobin < 8.0 g/dL) or a high-trigger (hemoglobin < 9.7 g/dL) group for RBC transfusion. Near-infrared spectroscopy assessed intraoperative oxygen desaturation in brain and muscle. Explorative outcomes included nationwide registry data on death and major vascular complications. The primary outcome, mean hemoglobin within 15 days of surgery, was significantly lower in the low-trigger group, at 9.46 vs 10.33 g/dL in the high-trigger group (mean difference, −0.87 g/dL; P = .022), as were units of RBCs transfused (median [interquartile range (IQR)], 1 [0-2] vs 3 [2-6]; P = .0015). Although the duration and magnitude of cerebral oxygen desaturation increased in the low-trigger group (median [IQR], 421 [42-888] vs 127 [11-331] minutes × %; P = .0036), muscle oxygenation was unaffected. The low-trigger group associated to a higher rate of death or major vascular complications (19/29 vs 8/29; hazard ratio, 3.20; P = .006) and fewer days alive outside the hospital within 90 days (median [IQR], 76 [67-82] vs 82 [76-84] days; P = .049). In conclusion, a perioperative protocol restricting RBC transfusion successfully separated hemoglobin levels and RBC units transfused. Exploratory outcomes suggested potential harm with the low-trigger group and warrant further trials before such a strategy is universally adopted. This trial was registered at www.clinicaltrials.gov as #NCT02465125.
Introduction
Fluid resuscitation with red blood cells (RBCs) plays a fundamental role in maintaining tissue oxygenation during blood loss. This is particularly relevant for the vascular surgical patient because of frequent preoperative anemia1,2 and surgical hemorrhage exceeding 500 mL,3-5 both of which are associated with improved survival if RBC transfusion is initiated during surgery compared with no transfusion.6 Furthermore, as these patients often present with cardiac disease,7-9 they may be particularly vulnerable to a hemoglobin (Hb) level below 10 g/dL.2,10 However, as allogeneic RBC transfusions are also associated with mortality and cardiovascular morbidity,11-13 and are an expensive and limited resource, randomized trials are warranted to balance risks and benefits of transfusion strategies in vascular surgery.14 In other surgical specialties, it has been demonstrated that withholding transfusion until reaching a Hb level of 7.0 to 8.0 g/dL is safe,15-19 but the majority of the evidence is based on postoperative stable patients. Moreover, previous trials involving blood loss above 500 mL lacked adequate separation of Hb levels and RBC units transfused during surgery,4,16,18,20 possibly as a result of the recruitment of patients with subsequent minimal blood loss or poor protocol adherence. Thus, the safety and feasibility of restricting RBCs during many types of surgeries remain unsettled.
This led us to design the Transfusion in Vascular surgery (TV) trial, as previously reported.21 We enrolled patients ahead of surgery. Intraoperative fluid therapy was standardized, using changes in cardiac stroke volume to guide when intravascular volume was adequate. An enrichment strategy secured that only patients with an Hb drop below 9.7 g/dL were randomly assigned to 1 of 2 Hb triggers for RBC transfusion. This ensured that patients with limited blood loss and/or high preoperative Hb were excluded. The objective was to assess whether a perioperative strategy that aimed to restrict RBC transfusion to an Hb drop below 8 g/dL (5 mmol/L, low-trigger), as compared with an Hb below 9.7 g/dL (6 mmol/L, high-trigger), would separate postoperative Hb levels and units of RBCs transfused, as well as influence intraoperative peripheral tissue oxygenation. We also report the occurrence of nonadherence, protocol suspensions, and as an exploratory outcome, death and major vascular complications.
Patients, material, and methods
Trial design and oversight
The TV trial was an investigator-initiated, single-center, stratified, parallel-group, patient- and partly assessor-blinded clinical trial with central web-based randomization. The protocol complies with the criteria outlined in the Declaration of Helsinki (7th revision, 2013) and was approved by the Scientific Ethical Committee of Region Zealand (Project-ID: SJ-426) and the Danish Data Protection Agency. The trial was registered at www.clinicaltrials.gov (NCT02465125) before enrolment of the first patient. Methods and plan for statistical analyses were published previously in detail in a protocol adhering to The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)-guideline.21 During trial enrolment, we observed that the number of patients randomly assigned after surgery was higher than expected. To reach the required sample size for the intraoperative outcome measures, we increased the sample size from 50 to 58 patients after the Ethical Committee’s approval. The setting was a vascular unit, servicing a population of 820 000, corresponding to approximately 15% of the Danish population.
Patients
Patients older than 40 years, who were referred for elective open infra-renal abdominal aortic aneurism (AAA) repair or lower limb bypass (infra-inguinal arterial bypass surgery or femuro-femoral crossover surgery) were asked to participate at the time of scheduling for their operation. Potential patients were excluded if they refused RBC transfusion, had a previous serious adverse reaction with blood products, had previously participated in the TV trial, or were unable to understand the benefits and risks of participating.
Randomization, blinding, and data collection
After preoperative informed and signed consent, randomization and implementation of the group assignment occurred on Hb drop below 9.7 g/dL at any time between the day before surgery and the 30th postoperative day. Randomization was performed by means of an external, centralized, web-based system at The Copenhagen Trial Unit. The allocation sequence was computer-generated in a 1:1 ratio with fixed block sizes of 6 stratified for type of surgery: open AAA operation vs lower limb bypass. Patients, statisticians (P.W. and J.C.J.) and outcome assessor (D.H.) were blinded to the group assignment. Tissue oxygenation, as assessed by near-infrared spectroscopy (NIRS; INVOS5100C; Somanetics, Troy, MI), was blinded to prevent access to data during anesthesia. NIRS is considered a trend monitor22 of regional Hb oxygen saturation. With a NIRS probe applied on the right part of the forehead, regional cerebral oxygenation (rScO2) was determined. Another probe was applied over the middle part of the right brachial biceps muscle to assess regional muscle oxygenation (rSmO2). Baseline was established ahead of surgery and was defined as the NIRS reading on reaching normovolemia (defined in “Surgery, anesthesia, and fluid”).
Cardiac troponin-I and P-creatinine were assessed the first and second day after randomization. Electrocardiogram was obtained preoperatively, in the case of troponin-I above 45 ng/L, and at follow-up 30 days after surgery. At the same follow-up time, patients had Hb measured and were contacted by telephone to inquire whether they had experienced symptoms related to ischemic heart disease or had been aware of their random assignment. Registry follow-up for the exploratory outcomes was requested half a year after surgery of the last randomized patient.
Surgery, anesthesia, and fluid
Surgery adhered to national guidelines and was performed by, or directly supervised by, a consultant in vascular surgery. All patients received sevoflurane, fentanyl, and rocuronium for general anesthesia. Patients with AAA repair had an epidural catheter inserted for perioperative analgesia. Each milliliter of blood loss was replaced by 2 to 3 mL Ringer’s acetate according to national guidelines.21 If blood loss exceeded 1000 mL, or total crystalloid administration was above 3000 mL, human albumin (5%) was administered until the Hb decreased below the allocated threshold for RBC transfusion. Fluid therapy was guided by optimization of cardiac stroke volume, using arterial waveform analysis (FloTrac ver.4.0; Edwards LifeSciences, Irvine, CA). When a fluid bolus increased stroke volume by less than 10%, the patient was considered normovolemic.23 Then, adequate perfusion pressure was ensured by keeping mean arterial pressure above 65 mm Hg with continuous infusion of norepinephrine (100 µg/mL). Use of plasma and platelets was guided by rotational thromboelastometry (ROTEM).24 Further details, including targets for heart rate, ventilation, oxygenation, and coagulation, are provided in the protocol.21
Intervention, protocol suspension, and nonadherence
After randomization, patients in the low-trigger group (experimental intervention) awaited RBC transfusion until Hb dropped below 8.0 g/dL (5 mmol/L) and received additional RBC units as needed to maintain an Hb level at or above 8.0 g/dL. Patients in the high-trigger group (control intervention) received 1 RBC unit immediately after randomization and additional units as needed to maintain an Hb level at or above 9.7 g/dL (6 mmol/L). The Hb level was measured every half hour during ongoing surgical bleeding, after every RBC transfusion, at a minimum on day 1 and 2 after surgery and randomization, and when clinically indicated. All RBC transfusions were allogeneic, leucoreduced, and nonradiated.
The assigned transfusion strategy was to be followed until 30 days after surgery, as well as in case of readmission. Physicians could temporarily suspend the protocol in case of uncontrollable bleeding, hypotension (mean arterial pressure <65 mm Hg) unresponsiveness to fluid replacement, stroke, or occurrence of ischemia in extremities or intestines. RBC transfusion could also be omitted in the case of suspected decompensated heart failure.
The term nonadherence was based on 2 events: an RBC unit administered at an Hb level above the allocated threshold or on indications not defined in the protocol suspensions above, and nonadherent failure to transfuse when the Hb level was below the allocated threshold.
Outcomes
The primary outcome was a longitudinal outcome of mean postoperative Hb days 0 to 15, in which day 0 was the Hb measured immediately after surgery on arrival at the postanesthesia care unit. The secondary outcomes were units of RBCs transfused, randomization rate, proportion of patients with protocol suspensions, adherence to Hb concentrations used for transfusion triggers (definitions in Table 2), intraoperative tissue oxygenation as determined by NIRS, and severe adverse events within 30 days of surgery. The NIRS outcome was the lowest rScO2 and lowest rSmO2 before an RBC transfusion (or at the end of operation if no transfusion was given). The duration and magnitude of cerebral desaturation from baseline, the desaturation load, was defined as the cumulative area (minutes × %) below baseline. Definitions of severe adverse events at day 30 are provided in supplemental Table 4, available on the Blood Web site.
Based on The Danish National Patient Registry,25 a 90-day exploratory outcome measure was added. This included death or major vascular complications, which encompassed severe adverse transfusion reaction (definition provided in the legend of Figure 4), acute myocardial infarction (adhering to the universal definition26 ), stroke, new-onset renal replacement therapy, unscheduled vascular surgery registered as secondary to the index operation, and major or minor amputation of the lower limb, defined as above or below the knee, or as forefoot or digit, respectively. The specific registry coding used to identify the major vascular complications is provided in supplemental Table 7. Days alive outside hospital within 90 days of surgery was also reported as an exploratory outcome to capture a net benefit/harm of the intervention (ie, death or hospital stay from any cause).
Statistical methods
With 50 randomized patients, a maximal type 1 error risk of 5%, and standard deviations as described previously,21 the trial had 95% power to show a difference in postoperative Hb of 1.6 g/dL (1.0 mmol/L) with 44 patients, 80% power to show a difference of 600 mL of RBC volume transfused with 46 patients, and 80% power to detect a difference in NIRS-determined rScO2 of 6% with 44 patients. Last, we also would be able to produce a 97.5% confidence interval (CI) equal to the adherence proportion plus or minus 8%. The required sample size for the desaturation load could not be estimated because of a lack of background data.27
All analyses were based on the intention-to-treat population and adjusted for age and type of surgery.28 Postoperative Hb (longitudinal outcome) was analyzed with generalized estimated equations (GEE), using STATA (version 15/MP). SAS (version 9.4) was used for all other analyses. Binary outcomes were analyzed using logistic regression, and odds ratios were converted to relative risks (RRs). Continuous outcomes were analyzed using linear regression and were additionally adjusted for the baseline value. We applied van Elteren’s test on count or skewed distributed data adjusted for type of surgery. Time to event was analyzed using Cox proportional hazards model and included death or major vascular complications occurring between randomization and right censoring (90 days after the last randomization, March 8, 2017). We tested the proportional hazards assumption for all covariates.29 Only 2-sided tests were used, and a P < .05 was considered statistically significant except for the NIRS hypothesis, where we had adjusted the significance level to P < .02 because of multiple comparisons of correlated outcome measures. An exploratory longitudinal analysis of the Hb data was performed, using the mixed-model, as this is unbiased when data are missing at random, as opposed to GEE, which uses the last observation carried forward. R studio (version 1.1.447) was used for generating graphs and tables. Further details are provided in the statistical analysis plan.21
Results
Study population
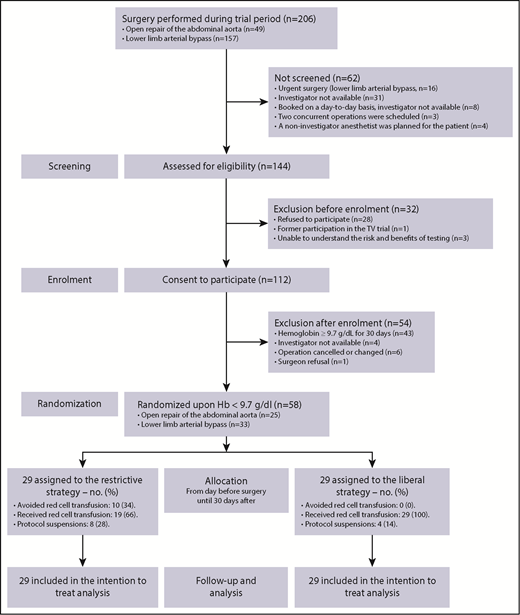
Between July 2015 and December 2016, 112 (78%) of 144 approached patients consented to participate. Eleven patient enrollments were discontinued before surgery (reasons provided in Figure 1). Randomization occurred on Hb decrease below 9.7 g/dL in 58 (57%) of the remaining 101 patients. This yielded a randomization rate of 58/144 (40%; 95% CI, 32%-48%) over the course of 17 months, allocating 29 to each group (Figure 1). This rate was lower than anticipated,21 which may be explained by an unexpected 20% to 25% reduction in patients referred for AAA repair during the trial period and by the reasons presented in Figure 1. There was no patient drop-out during follow-up. Registry data were provided 9 months after the last randomization. NIRS data were subsequently extracted, which completed the database in October 2017.
Screening, enrolment, randomization, and follow-up. Randomization occurred in 46 (79%) patients before end of surgery, in 53 patients (91%) within or on the second postoperative day, and in the remaining 5 patients on the third, fourth, sixth, ninth, and fifteenth day.
Screening, enrolment, randomization, and follow-up. Randomization occurred in 46 (79%) patients before end of surgery, in 53 patients (91%) within or on the second postoperative day, and in the remaining 5 patients on the third, fourth, sixth, ninth, and fifteenth day.
Patient characteristics were similar at baseline (Table 1). The stratification distributed surgical procedures equally between the 2 groups. Further surgery specifics are provided in the supplementary material (supplemental Table 1). At day 30 follow-up, 98% of the patients were unaware of or could not recollect their assigned transfusion threshold.
Primary outcome measure
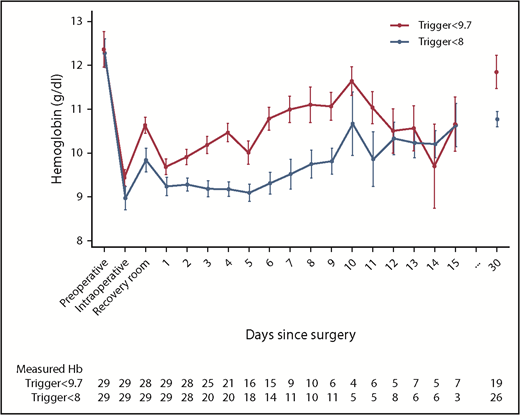
Within 15 days of surgery, mean postoperative Hb was 9.46 g/dL in the low-trigger group vs 10.33 g/dL in the high-trigger group. This difference was statistically significant with GEE (P = .022) and when the mixed-model was applied (mean difference, −0.91 g/dL; 95% CI, −1.21 to −0.61; P < .001). The mean Hb difference was pronounced on day 0 in the 46 patients randomly assigned before the end of surgery (−1.35 g/dL; P < .001) and at follow-up 30 days after surgery (−1.07 g/dL; P = .004; Table 2; Figure 2; supplemental Figures 1 and 2). These results were consistent across subgroups and sensitivity analyses (supplemental Table 2).
Hb levels in randomized patients at baseline and after vascular surgery. The graph shows the mean daily lowest Hb measurement between the day before surgery (preoperative), during surgery (intraoperative), on arrival in the recovery room (or intensive care unit), and on the first to fifteenth postoperative day followed by the Hb level at follow-up (day 30). Bars indicate ± standard error of the mean. The table below shows the number of patients with available Hb measurements each day. A graph with a separate panel for patients randomized before and after surgery are presented in supplemental Figure 2.
Hb levels in randomized patients at baseline and after vascular surgery. The graph shows the mean daily lowest Hb measurement between the day before surgery (preoperative), during surgery (intraoperative), on arrival in the recovery room (or intensive care unit), and on the first to fifteenth postoperative day followed by the Hb level at follow-up (day 30). Bars indicate ± standard error of the mean. The table below shows the number of patients with available Hb measurements each day. A graph with a separate panel for patients randomized before and after surgery are presented in supplemental Figure 2.
Secondary outcome measures
The number of RBC units transfused was significantly reduced with the low-trigger strategy, overall (median [interquartile range (IQR)], 1 [0-2] vs 3 [2-6]; P = .0015) and during and after surgery (Table 2). The proportion of patients exposed to RBCs was reduced by 34%, and the overall usage of RBC units was more than halved (Table 2). Although nonadherent RBC transfusions seemed more common in the low-trigger group, there was a clear trend toward more cases of nonadherent failure to transfuse in the high-trigger group. Overall, any nonadherence occurred in 28% and 34% of the patients in the 2 groups (Table 2; supplemental Table 2.6). Apart from the intraoperative infused volume of RBCs, parameters related to intraoperative fluid balance and duration of anesthesia, surgery, and cross clamp were similar in the 2 groups (supplemental Table 1). The overall mean blood loss was 1268 mL, but no intraoperative ROTEM assessments indicated need for plasma or platelet substitution.
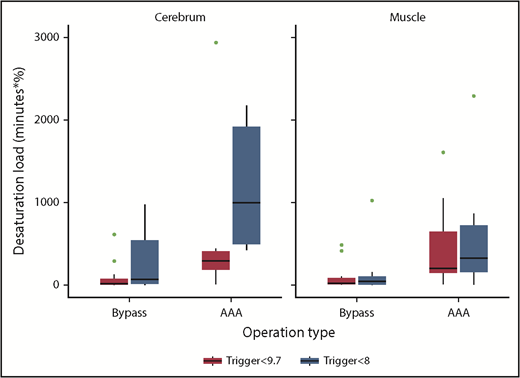
The cerebral desaturation load was significantly higher in the low-trigger group (median [IQR], 421 [42-888] minutes × % vs 127 [11-331] minutes × %; P = .0036; Table 3; Figure 3). The muscle desaturation load and the lowest rScO2 and rSmO2 values before an RBC transfusion did not differ significantly. Square root transformation of the desaturation load (to approximate a normal distribution for linear regression; supplemental Table 3.1) and the sensitivity analysis (excluding patients who did not receive RBC transfusions; supplemental Table 3.2) showed similar point estimates. Within 30 days after the operation, both troponin-I (≥45 ng/L) and P-creatinine (≥26.5 µg/L) had occurred in approximately 33% of patients in both groups, and the composite of any serious adverse events did not differ with statistical significance (RR, 0.74; 95% CI, 0.42-1.30; P = .28; supplemental Table 4).
Cerebral and biceps muscle desaturation load stratified by type of surgery. Bypass, lower limb arterial bypass. The desaturation load was defined as the cumulative area (minutes × %) below the baseline tissue oxygenation reading (as determined by near-infrared spectroscopy).
Cerebral and biceps muscle desaturation load stratified by type of surgery. Bypass, lower limb arterial bypass. The desaturation load was defined as the cumulative area (minutes × %) below the baseline tissue oxygenation reading (as determined by near-infrared spectroscopy).
Exploratory outcomes
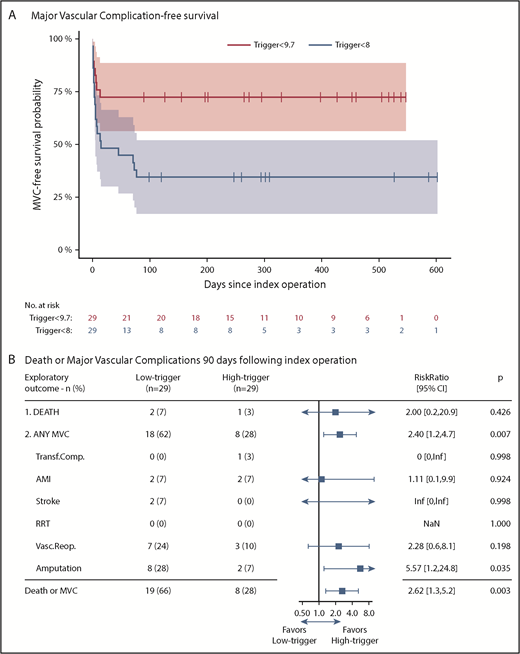
At day 90 after surgery, 2 patients had died in the low-trigger group vs 1 in the high-trigger group (RR, 2.00; P = .43). Major vascular complications occurred in significantly more patients allocated to the low-trigger group (18/29 vs 8/29; RR, 2.40; P = .007; Figure 4B). Cox regression analysis of time to death or major vascular complication corroborated this finding (hazard ratio, 3.20; P = .006; Figure 4A). There was no subgroup heterogeneity between surgery type and transfusion trigger groups (test of interaction, P = .97), and the stratified Kaplan-Meier plot also demonstrated similarly increased hazards with the low-trigger strategy regardless of operation type (supplemental Figure 4). Post hoc Cox regressions excluding minor amputations and/or reoperations supported the findings (supplemental Table 9). Last, the low-trigger group had significantly fewer days alive outside of hospital within the 90 days after the operation (median [IQR], 76 [67-82] days vs 82 [76-84] days; P = .049; supplemental Table 6).
Major vascular complication-free survival and relative risk for death or major vascular complications at 90 days. (A) Major vascular complication-free survival probability with 95% CI in the intention-to-treat population. Data were censored 90 days after the last randomized patient. (B) shows the relative risks (blue boxes) with 95% CIs (horizontal lines) for the exploratory outcome measures death or major vascular complications at day 90 in the low-trigger group as compared with the high-trigger group. Low-trigger, Hb lower than 8 g/dL; high-trigger, Hb lower than 9.7 g/dL. MVC, major vascular complication. Transf.Compl., severe adverse transfusion reaction (anaphylactic reaction, transfusion-associated circulatory overload, transfusion-related acute lung injury within 6 hours after RBC transfusion or severe acute hemolytic transfusion reaction within 24 hours after RBC transfusion); see trial protocol21 for further detail. AMI, acute myocardial infarction. RRT, renal replacement therapy. Vasc.Reop., vascular reoperation (specifications provided in supplemental Table 8). Amputation, lower limb amputation from femur to toes. When considering major amputations (femoral or crural) at day 90: 3 (10%) major amputations had occurred in the low-trigger group vs zero (0%) in the high-trigger group; and at right censoring: 7 (24%) vs zero (0%), respectively. *Unadjusted because of lack of model fit with zero events in 1 stratum. All other logistic regressions were adjusted for operation type and age. Odds ratios were converted to RR using the δ-method, where probabilities were derived from the coefficients of the logistic regression using the mean age (73 years) and operation type (0.43); 95% CI was calculated from the standard error.
Major vascular complication-free survival and relative risk for death or major vascular complications at 90 days. (A) Major vascular complication-free survival probability with 95% CI in the intention-to-treat population. Data were censored 90 days after the last randomized patient. (B) shows the relative risks (blue boxes) with 95% CIs (horizontal lines) for the exploratory outcome measures death or major vascular complications at day 90 in the low-trigger group as compared with the high-trigger group. Low-trigger, Hb lower than 8 g/dL; high-trigger, Hb lower than 9.7 g/dL. MVC, major vascular complication. Transf.Compl., severe adverse transfusion reaction (anaphylactic reaction, transfusion-associated circulatory overload, transfusion-related acute lung injury within 6 hours after RBC transfusion or severe acute hemolytic transfusion reaction within 24 hours after RBC transfusion); see trial protocol21 for further detail. AMI, acute myocardial infarction. RRT, renal replacement therapy. Vasc.Reop., vascular reoperation (specifications provided in supplemental Table 8). Amputation, lower limb amputation from femur to toes. When considering major amputations (femoral or crural) at day 90: 3 (10%) major amputations had occurred in the low-trigger group vs zero (0%) in the high-trigger group; and at right censoring: 7 (24%) vs zero (0%), respectively. *Unadjusted because of lack of model fit with zero events in 1 stratum. All other logistic regressions were adjusted for operation type and age. Odds ratios were converted to RR using the δ-method, where probabilities were derived from the coefficients of the logistic regression using the mean age (73 years) and operation type (0.43); 95% CI was calculated from the standard error.
Discussion
In a carefully designed trial with thorough clinical follow-up and statistical evaluation, we aimed to assess the effects of different Hb trigger levels for RBC transfusion while respecting established concepts for intraoperative fluid resuscitation.30 We observed that for patients undergoing elective AAA or lower limb bypass surgery, a perioperative protocol aiming to restrict RBC transfusion until Hb had reached a level below 8 vs 9.7 g/dL, significantly separated postoperative Hb levels. Importantly, the overall use of RBC units was more than halved. The lowered Hb levels also reflected an increased cerebral desaturation load during surgery compared with the high-trigger strategy. The trial was not powered to detect differences in patient important outcomes, but data suggested a higher rate of death or major vascular complications in patients allocated to the low-trigger group.
Several recent recommendations support RBC transfusion at an Hb of 7 to 8 g/dL in most patients.31-34 This strategy also seems adopted by the Society of Vascular Surgery,35 which suggests a threshold of 7 g/dL during or after AAA repair in the absence of rapid ongoing blood loss, while acknowledging the need for trials on RBC, plasma, and platelet transfusion strategies. There are, however, several crucial differences between the available trials that inform RBC transfusion practice and transfusion in vascular surgery. First, most of the evidence is based on postoperatively stable patients with limited surgical hemorrhage, as outlined here. Second, cardiovascular disease (CVD) is particularly prevalent in vascular surgery. In the consecutively screened and unselected TV trial population, 80% to 90% of randomized patients had CVD at baseline compared with 63% in the FOCUS trial, using the same definitions.15 The FOCUS trial demonstrated that RBC transfusion at Hb below 8 was as safe as 10 g/dL after hip fracture surgery, but inclusion criteria were widened during the trial to also enroll patients with risk factors for CVD, and the inclusion period was extended to 5 years, which raises concern of selection bias. Third, although the recent TRICSIII trial18,36 provides evidence that a restrictive transfusion strategy is safe, these patients are on cardiopulmonary bypass and have their CVD condition somewhat resolved during surgery, as opposed to the vascular surgery patients, who depend on their own ability to increase cardiac output subsequent to both hemorrhage and increased oxygen demand from surgical trauma. In addition, perioperative aggravation of the CVD condition is frequent in vascular surgery, as reported both in the TV trial and in several other cohorts.37-41
Recruiting patients for a randomized trial comparing strategies for resuscitating hemorrhage during surgery may be challenged by limited blood loss. In hip fracture, major abdominal, and cardiac surgery, the mean reported blood loss is around 200 to 500 mL.15,18,42 Preoperative randomization of all comers in such settings may include patients who would never have been considered for RBCs with either strategy as a result of limited Hb drop, which was the case in 22% to 27% of patients in previous cardiac surgery trials of RBC trigger levels.18,43 When bleeding does occur at higher volumes, patients are often excluded,44-46 or poor adherence may diminish group separation in terms of Hb levels and units of RBCs transfused.4,16,20 If randomization is postponed until after surgery, approximately 25% of patients will already have received un-protocolled transfusions.15,19,44 These details are important because if a clinically significant separation of Hb levels or RBC units transfused is not accomplished, any causal inference on the results becomes spurious. In the TV trial, we used a novel approach in which all enrolled patients received protocolled anesthesia, monitoring, and standardized fluid therapy.21,30 In addition, blood sampling ensured randomization immediately on an Hb decrease below 9.7 g/dL, whereby 43/112 patients were excluded because of limited blood loss or high preoperative Hb. With this so-called enrichment strategy,47 all randomized patients encountered a perioperative Hb below 9.7 g/dL. One drawback is a lower randomization rate (40%) than if we had applied preoperative randomization on all 112 consented patients (78%).
Despite the nonadherent events in both groups, and that 12 of 58 were randomly assigned after surgery, data showed significant separation of Hb levels and transfused units of RBCs consistently across all subgroups and sensitivity analyses. We also observed a long-term effect, as significant Hb separation was retained at 30 days follow-up. This was accomplished by using Hb as the main indicator for transfusion, which enables standardized intervention across all phases of hospital admission as a result of the ubiquity of point-of-care devices for Hb assessment. It may be argued that Hb measurement during surgery is imprecise because of the limited time for equilibration of fluid between the vascular beds and the interstitium. We mitigated this phenomenon by exclusively measuring the Hb level during normovolemia and not during uncontrollable bleeding or hypotension. It is our interpretation that the pronounced Hb separation observed in the recovery room demonstrates that this method is feasible and reliable. We acknowledge that other indicators for RBC transfusion may be used48,49 or are recommended during surgery,34 but they also need further validation in randomized clinical trials.31,32 The reduction in RBC units transfused observed with the low-trigger strategy indicates a potentially large cost-saving effect on RBC use, as major surgery, such as AAA repair, is among the most blood product-consuming procedures.50,51
The effect of transfusion strategy on tissue oxygenation was evaluated noninvasively by NIRS. Our findings were similar to what has been observed in experimental assessments of brain and muscle oxygen homeostasis during acute hemodilution.52 With the low-trigger strategy, the cerebral desaturation load increased, whereas the muscle oxygenation was unaffected. This could be explained by the fact that the muscles have a lower oxygen metabolism during anesthesia and surgery, and that muscle oxygenation mostly depends on cardiac output. Cardiac output seems to have been reasonably maintained in both groups, as urinary output (supplemental Table 1) was largely unchanged by different Hb triggers. Apart from cardiac output, rScO2 integrates the influence of CO2 and blood perfusion pressure on the brain.53 Cerebral perfusion pressure is vulnerable to carotid stenosis, which is frequent among vascular surgical patients,54,55 but the prevalence was not assessed in our patients. Also, alfa-adrenergic stimulation by norepinephrine may influence rScO2.56 The data are taken to reflect that brain was more vulnerable than muscle to the low-trigger strategy, and that oxygen delivery (cardiac output × blood oxygen content) did not meet the higher metabolic requirements for oxygen in the brain compared with the muscle.
Several reports acknowledge that lowered rScO2 relates to poor clinical outcome,57,58 but the clinical relevance of our finding is unknown. We did observe an increased risk for death or major vascular complications in patients allocated to the low-trigger strategy, which was notably a result of a higher occurrence of amputations in the patients with lower limb bypass. For patients with AAA allocated to the low-trigger strategy, both the occurrence of death and various major vascular complications was increased (supplemental Table 8). Importantly, the 2 groups were balanced in severity of lower limb artery disease at baseline (Table 1), and the specifics of surgery performed did also not indicate an imbalance (supplemental Table 1). Consistently, patients in the low-trigger group spent almost 1 week less alive outside of the hospital within 90 days. Although the complication rates seem high, it is essential to recall that our trial population was enriched with patients with low preoperative Hb and/or major surgical hemorrhage, and thus reflects a population with relatively high comorbidity burden and more complicated surgery than the general vascular surgery population.
Limitations of this trial are largely lack of blinding as a result of use of open-label design, which is inherent in trials on RBC transfusion. Second, a single-center trial has limited external validity, and it is important that the explorative outcome results are very cautiously interpreted, given the low sample size for detecting or rejecting even large effects, and for now, the explorative outcome results are just hypothesis-generating. Several strengths can be mentioned: the TV trial includes a prepublished protocol and statistical analysis plan, central randomization, assessor blinding, successful patient blinding, and to the best of our knowledge, this is the first transfusion trial using an enrichment strategy, which secures perioperative assessment of transfusion strategies while reducing random noise and heterogeneity through exclusion of patients who are unlikely to be considered for RBC transfusion.
In summary, this feasibility trial successfully separated perioperative Hb levels and units of RBCs transfused, but also demonstrated increased cerebral desaturation with the low-trigger strategy. A large randomized trial, with lowest possible risk for bias, assessing the safety of transfusion strategies in vascular surgery is essential before clinical practice guidelines settle for restrictive RBC transfusion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and the staff at the operation theater, vascular surgery, anesthetic, and high-dependency care and intensive care unit and blood bank, making the trial a reality. A special thanks to nurses Helle Witthøft Jørgensen and Izabella Ohm-Hieronymussen for helping with data collection during surgery.
This work was supported by the Local Research Fund of Region Zealand, Næstved, Slagelse, Ringsted Hospital (2015-01-26) (A. Møller), and from Region Zealand Health Research Fund (15-000342; A. Møller), Denmark.
A. Møller is enrolled as a PhD student at the University of Copenhagen. This work is submitted in partial fulfillment of the requirement for the PhD.
The funders of this study are public organizations and had no role in study design, collection, management, analysis, and interpretation of data, writing of the report, or the decision to submit the report for publication.
Authorship
Contribution: A. Møller is the principal investigator and initiator of the TV Trial and the main author of the protocol, the detailed statistical analysis plan, and the main manuscript, and provided a major contribution to the acquisition, analysis, and interpretation of data; H.B.N. provided a major contribution to the protocol design, interpretation of the data, and drafting of the main manuscript; J.W. provided a major methodological contribution to protocol design, the detailed statistical analysis plan, interpretation of the data, and drafting of the main manuscript; O.B.P. is a coauthor of the protocol and contributed to data acquisition and interpretation of the data; D.H. is a coauthor of the protocol and an investigator with substantial contributions to data acquisition and interpretation of the data, and performed blinded outcome assessment; P.W. is the primary statistician with a major contribution to the analysis and interpretation of data; and K.V.M., B.G.U.R., and A. Mortensen are investigators who made substantial contributions to data acquisition; J.C.J. is a statistician who coauthored the statistical analysis plan, performed analysis of the primary outcome measure by generalized estimated equations, and contributed to the interpretation of data; S.S. is a trial sponsor who contributed to participant recruitment and data acquisition; and all authors provided critical revision of the article and gave approval of the final submitted version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anders Møller, Department of Anesthesia and Intensive Care, Slagelse Hospital, Fælledvej 11, DK-4200 Slagelse, Denmark; e-mail: dr.andersm@gmail.com.