Key Points
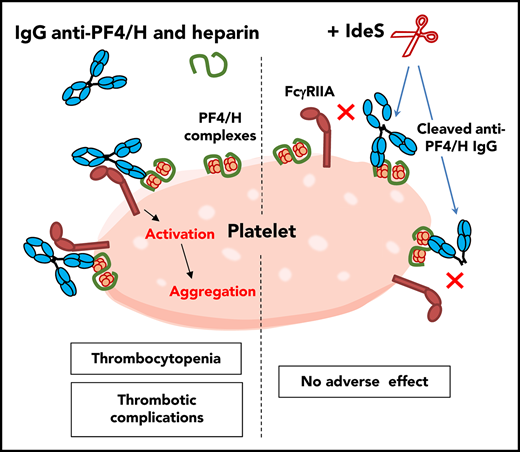
Cleavage of the anti-PF4/H antibody hinge region by IdeS abolishes FcγRIIA-dependent cellular activation.
IdeS treatment prevents thrombus formation and thrombocytopenia induced by anti-PF4/H antibodies.
Abstract
Heparin-induced thrombocytopenia (HIT) is due to immunoglobulin G (IgG) antibodies, which bind platelet factor 4 (PF4) modified by polyanions, such as heparin (H). IgG/PF4/polyanion complexes directly activate platelets via Fc gamma type 2 receptor A (FcγRIIA) receptors. A bacterial protease, IgG-degrading enzyme of Streptococcus pyogenes (IdeS), cleaves the hinge region of heavy-chain IgG, abolishing its ability to bind FcγR, including FcγRIIA. We evaluated whether cleavage of anti-PF4/H IgG by IdeS could suppress the pathogenicity of HIT antibodies. IdeS quickly cleaved purified 5B9, a monoclonal chimeric anti-PF4/H IgG1, which led to the formation of single cleaved 5B9 (sc5B9), without any reduction in binding ability to the PF4/H complex. However, as compared with uncleaved 5B9, the affinity of sc5B9 for platelet FcγRIIA was greatly reduced, and sc5B9 was also unable to induce heparin-dependent platelet activation. In addition, incubating IdeS in whole blood containing 5B9 or HIT plasma samples led to cleavage of anti-PF4/H antibodies, which fully abolished the ability to induce heparin-dependent platelet aggregation and tissue factor messenger RNA synthesis by monocytes. Also, when whole blood was perfused in von Willebrand factor–coated microfluidic channels, platelet aggregation and fibrin formation induced by 5B9 with heparin was strongly reduced after IdeS treatment. Finally, IdeS prevented thrombocytopenia and hypercoagulability induced by 5B9 with heparin in transgenic mice expressing human PF4 and FcγRIIA receptors. In conclusion, cleavage of anti-PF4/H IgG by IdeS abolishes heparin-dependent cellular activation induced by HIT antibodies. IdeS injection could be a potential treatment of patients with severe HIT.
Introduction
Heparin-induced thrombocytopenia (HIT) is a frequent drug-adverse event caused by platelet-activating antibodies (Abs) directed against complexes of platelet factor 4 (PF4) modified by polyanions such as heparin (H).1,2 Despite having thrombocytopenia, affected patients usually develop thrombosis, and this particular clinical presentation is explained by the central role of Fcγ receptors in the pathophysiology of HIT.3,4 Indeed, the HIT Ab immunoglobulin G (IgG) directly activates platelets and monocytes in the presence of heparin after Fc gamma type 2 receptor A (FcγRIIA) engagement.5-7 The cross-linking of FcγRIIA by immune complexes on the cell surface leads to ordered receptor clustering favoring the phosphorylation of tyrosine residues by Src kinases within the immunoreceptor tyrosine-based activation motif of FcγRIIA, which results in platelet activation with granule content secretion and platelet aggregation.8-10
Importantly, the binding of IgG to Fcγ receptors, including FcγRIIA, involves a highly conserved stretch of amino acids within the lower hinge/CH2 region of γ heavy chains.11,12 Several bacterial proteases, including glutamyl endopeptidase V8 of Staphylococcus aureus, IgG-degrading enzyme of Streptococcus pyogenes (IdeS), and human proteases (ie, matrix metalloproteinases 3, 7, and 12 and cathepsin G) can cleave the lower hinge/CH2 region of IgG.13-15 This IgG cleavage is a sequential process, whereby single cleaved IgG (scIgG), resulting from the cleavage of one heavy chain, is rapidly generated as an intermediate product before the cleavage of the second heavy chain to obtain F(ab′)2. Of note, scIgGs show strongly reduced affinity for Fcγ receptors (including FcγRIIA and FcγRIIIA), and they no longer induce Ab-dependent cellular cytotoxicity.16
In several studies, IdeS was shown to rapidly and effectively cleave all endogenous IgGs in human blood in vitro.17-19 In addition, a single injection of IdeS in a mouse model of immune thrombocytopenic purpura, induced by the administration of rabbit antiplatelet Abs, led to rapid correction of platelet count.20 Recently, to prevent acute Ab-mediated transplant rejection, IdeS was administered 4 to 6 hours before incompatible renal transplantation to patients with anti-human leukocyte antigen Abs.21 The results demonstrated complete cleavage of circulating IgG before the surgical procedure and successful transplantation in 24 of the 25 treated patients.
The management of severely affected HIT patients may be very difficult in specific clinical conditions, such as cardiac surgery, and alternative non-heparin treatments may be inefficient and dangerous.22 Since FcγR–IgG interactions are critical in the pathogenesis of HIT, we investigated whether IdeS could be a good candidate as a new therapeutic approach in HIT. In this study, we showed that IdeS could cleave anti-PF4/H Abs, including 5B9, a monoclonal anti-PF4/H Ab with a human IgG1 Fc fragment. IdeS greatly reduced the ability of anti-PF4/H Abs to activate platelets in vitro and suppressed the pathogenicity of 5B9 in vivo.
Materials and methods
5B9 was used as an HIT Ab model because we recently demonstrated that this chimeric anti-PF4/H monoclonal IgG1 Ab fully mimicked human HIT Abs.23 Fluorescence in situ hybridization–conjugated monoclonal Ab anti-CD32 (clone IV.3) was from StemCell Technologies. IdeS with low endotoxin (<0.1 EU/vial of 5000 U) was from Genovis. Purified human von Willebrand factor (VWF) was a gift from Cécile Denis (UMR U1176) and unfractionated heparin (UFH; Heparin Choay) was from Sanofi. Adenosine diphosphate (ADP) (ELITech Group), thrombin receptor-activating peptide (Agro-bio), thrombin (Stago), and Horm type I collagen (Takada) from equine tendon were used for functional platelet assays. Western blot reagent and lipopolysaccharide (LPS) were from Sigma-Aldrich.
Proteolytic cleavage of purified 5B9 by IdeS
Kinetic studies of 5B9 cleavage were performed using purified 5B9 (1 mg/mL) incubated with IdeS (0.02 U/µg IgG) at 37°C, and its activity was stopped by iodoacetamide (10 mM). Cleavage of 5B9 by IdeS was assessed by 6% nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining with Coomassie blue. The relative amounts of intact 5B9, sc5B9, and 5B9 F(ab′)2 were evaluated in each sample using ImageJ software. Finally, for functional assays, IdeS (molecular weight, 35 kDa) and iodoacetamide were removed from IgG fractions obtained after a 6-minute cleavage by IdeS by using a Vivaspin 50 kDa column (Sartorius).
ELISA for Abs to PF4/H complexes
The binding of IdeS-treated 5B9 to PF4/H complexes was evaluated using Asserachrom HPIA IgG according to the manufacturer’s recommendations. Additional ELISA experiments were also performed using a secondary Ab specific for the murine κ chain (Jackson ImmunoResearch)
Platelet activation and aggregation tests
Whole blood (WB) from healthy donors was collected according to the International Society on Thrombosis and Haemostasis recommendations24 and after informed consent and approval by the university ethics committee.
Serotonin release assays were performed as described elsewhere25 with 5B9 or IdeS-treated 5B9 in the presence of different concentrations of UFH (0, 0.01, 0.1, 0.5, 1, and 10 IU/mL) using washed platelets adjusted to 350 × 106/mL.
WB from healthy donors was also collected on 0.129 M sodium citrate, and platelet-rich plasma was isolated by centrifugation at 200g for 15 minutes before aggregation tests with 5B9 or IdeS-treated 5B9 (50 µg/mL) in the presence of UFH (0.5 IU/mL) using an APACT 4 aggregometer (ELITech Group).
WB impedance aggregometry assay was adapted from Morel-Kopp26 and involved use of a multiplate analyzer (Roche). Briefly, 5B9 (20 µg/mL) or HIT plasma sample (1:8 final dilution) was added in healthy donor WB on hirudin (15 µg/mL). Samples were incubated without or with IdeS for 6 minutes before the addition of UFH (1 IU/mL). Changes in impedance were recorded over 15 minutes. Assays evaluating platelet responses to ADP and collagen without or with IdeS were performed as controls. Similar experiments were performed to evaluate the cleavage of total IgG and anti-PF4/H IgG in WB, and IdeS activity was inhibited by using iodoacetamide (10 mM) after 6-minute incubation. Plasma samples were obtained from WB by centrifugation at 2000g for 15 minutes.
Flow cytometry analysis of sc5B9 binding to platelet FcγRIIA
Washed platelets (5 × 104 in 10 μL) were incubated with varying concentrations of 5B9 or IdeS-treated 5B9 (30 minutes) in the presence of UFH (0.1 IU/mL). Platelets were incubated with fluorescence in situ hybridization–conjugated IV.3 (1 μg/mL, 30 minutes) and analyzed by flow cytometry. The results were expressed as the percentage of inhibition of IV.3 binding as described previously.6
Proteolytic cleavage of anti-PF4/H by IdeS in WB
To further demonstrate the specific cleavage of anti-PF4/H Abs, WB from healthy donors containing 5B9 or the plasma from 3 HIT patients was incubated with or without various concentrations of IdeS. After centrifugation, plasma samples obtained were incubated after dilution for 1 hour in PF4/H-coated wells (Asserachrom, Stago). After washings, 50 µL Laemmli buffer 1× was added for 30 minutes at 37°C to elute bound Abs from PF4/H complexes. Collected samples were submitted to 6% SDS-PAGE, and after transfer onto nitrocellulose membranes, anti-PF4/H IgG fragments were detected by immunoblotting with horseradish peroxidase–conjugated anti-human γ chain polyclonal Abs and imaged with a Fusion FX (Vilber).
Assay of TF and CD14 mRNA levels after WB stimulation with 5B9
WB collected from healthy donors on 0.129 M sodium citrate was incubated 6 minutes at 37°C with 5B9 (50 µg/mL) or LPS (1 µg/mL) with or without IdeS (0.02 U/µg IgG) before the addition of H (0.5 IU/mL). After 60 minutes, total RNA was isolated from cells using the Qiamp RNA blood mini kit (Qiagen), and tissue factor (TF) and CD14 (control gene) messenger RNA (mRNA) levels were measured by quantitative polymerase chain reaction as described elsewhere.6
Analysis of microfluidic thrombosis
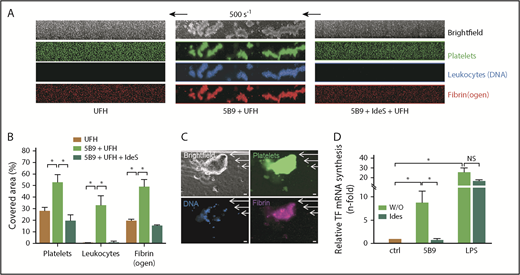
WB collected on 0.129 M sodium citrate was incubated for 6 minutes at 37°C with 5B9 (40 µg/mL) with or without IdeS (0.02 U/µg IgG) before the addition of H (0.5 IU/mL). After 30 minutes at 37°C, blood samples were recalcified to 5 mM CaCl2 and perfused at a shear rate of 20 µL/min (500 s−1; 20 dyn/cm2) at 37°C in microfluidic channels (Vena8 Fluo+, Cellix) precoated overnight at 4°C with 40 µg/mL human vWF. Platelet and fibrin deposition was visualized by adding DiOC6 (10 µM) and Alexa Fluor 647–labeled fibrinogen (100 µg/mL; Invitrogen) to WB. Leukocytes were labeled by using a specific DNA dye (Hoechst 33342). Images were acquired every 3 seconds for 5 minutes using an Axio Observer Z1 microscope (Zeiss) and an EC Plan-Neofluar 20×/0.5 Ph2 M27 objective equipped with an ORCA-R2 C10600 digital CCD camera (Hamamatsu) controlled by Zen 2012 image-capture software. At the end of each experiment, channels were washed with phosphate-buffered saline (PBS)/EDTA and fixed with 2% paraformaldehyde (BD Biosciences). Postflow fibrin staining was done using a fibrin-specific monoclonal Ab (clone 59D8)27 coinjected with Cy3-labeled anti-mouse F(ab′)2 (Jackson ImmunoResearch). The percentage of surface covered by aggregates was measured using ImageJ software and after analyzing at least 3 different areas for each experimental condition.
Study in HIT mouse model of the effect of IdeS on thrombocytopenia and hypercoagulability induced by 5B9
All studies in transgenic HIT mice (human FcγRIIA, hPF4-transgenic, mPF4 knockout)28 were performed in accordance with the guidelines and approval of the institutional animal care and use committees of Thomas Jefferson University. The animal facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. One week before experiments, platelets were counted for baseline values. Mice were injected with 5B9 intraperitoneally (15 µg/g body weight; n = 6) on day 0. On day 1, IdeS (25 U/g; n = 3) or PBS (n = 3) was administered via retro-orbital injection using 28G1/2 needles. H (1.4 U/g) was then injected subcutaneously 2, 24, and 48 hours later. Platelets were counted 4 hours after each injection of heparin using a Hemavet Analyzer (model 850, CDC Technologies). Plasma concentrations of thrombin–antithrombin complexes (TATs) were also measured as a marker of thrombin generation in vivo by enzyme-linked immunosorbent assay as described previously.29
Statistical analysis
Student t test was used to compare data obtained in vitro and in vivo after IdeS treatment under different conditions. P < .05 was considered statistically significant.
Results
IdeS rapidly cleaves 5B9 and inhibits its functional activity
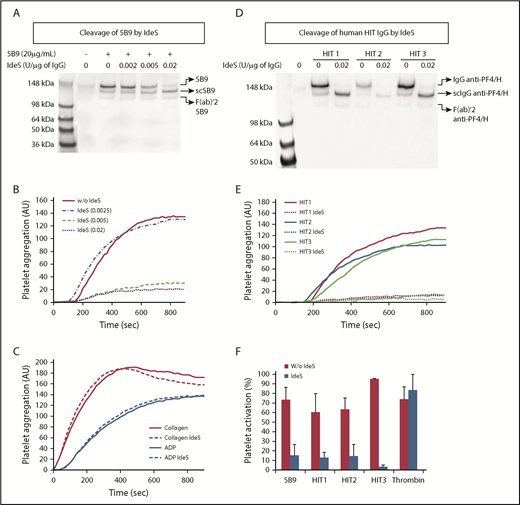
IdeS rapidly cleaved purified 5B9. Indeed, 60% and 79% of the IgG fragments analyzed were sc5B9 after 3 and 6 minutes of incubation, respectively, but the relative amount of F(ab′)2 was weak (<10%; Figure 1A-B). The cleavage of the second heavy chain leading to 5B9 F(ab′)2 was significant after 30 minutes and completed after overnight incubation (21% and 66%, respectively; Figure 1A-B). The binding to PF4/H complexes of 5B9 cleaved by IdeS during 3 to 180 minutes was unchanged compared with that of untreated and intact 5B9 IgG and slightly decreased only after overnight incubation with the protease (Figure 1C).
Cleavage of purified 5B9 by IdeS. Representative Coomassie blue SDS-PAGE (n = 3 experiments) (A) and percentages of intact 5B9 IgG, single cleaved 5B9 (sc5B9), and F(ab′)2 5B9 (mean values, n = 3), as defined by ImageJ, after incubation of 5B9 with IdeS (0.02 U/µg IgG) at 37°C (B). (C) Binding of cleaved 5B9 (1 µg/mL) to PF4/H complexes, as evaluated by ELISA with a secondary Ab specific to murine K chain (mean ± standard error of the mean [SEM], n = 2 experiments). A, absorbance; O/N, overnight. (D) Inhibition of IV.3 binding on platelets with increasing concentrations of 5B9 or sc5B9 (mean ± SEM; n = 3 platelet donors). (E) Representative platelet aggregation curve (n = 2 experiments) with untreated 5B9 (50, 25, 12.5, and 6.25 µg/mL) or sc5B9 (50 µg/mL) added to platelet-rich plasma with UFH (0.5 IU/mL). (F) Representative profile of serotonin release assay (n = 2 experiments) with untreated 5B9 (20 µg/mL) and sc5B9 (20 µg/mL) and different concentrations of UFH. (D-F) Single cleaved 5B9 (sc5B9) is the IgG fraction obtained after incubating 5B9 with IdeS (0.02 U/µg IgG; 6 minutes) at 37°C. *P < .05.
Cleavage of purified 5B9 by IdeS. Representative Coomassie blue SDS-PAGE (n = 3 experiments) (A) and percentages of intact 5B9 IgG, single cleaved 5B9 (sc5B9), and F(ab′)2 5B9 (mean values, n = 3), as defined by ImageJ, after incubation of 5B9 with IdeS (0.02 U/µg IgG) at 37°C (B). (C) Binding of cleaved 5B9 (1 µg/mL) to PF4/H complexes, as evaluated by ELISA with a secondary Ab specific to murine K chain (mean ± standard error of the mean [SEM], n = 2 experiments). A, absorbance; O/N, overnight. (D) Inhibition of IV.3 binding on platelets with increasing concentrations of 5B9 or sc5B9 (mean ± SEM; n = 3 platelet donors). (E) Representative platelet aggregation curve (n = 2 experiments) with untreated 5B9 (50, 25, 12.5, and 6.25 µg/mL) or sc5B9 (50 µg/mL) added to platelet-rich plasma with UFH (0.5 IU/mL). (F) Representative profile of serotonin release assay (n = 2 experiments) with untreated 5B9 (20 µg/mL) and sc5B9 (20 µg/mL) and different concentrations of UFH. (D-F) Single cleaved 5B9 (sc5B9) is the IgG fraction obtained after incubating 5B9 with IdeS (0.02 U/µg IgG; 6 minutes) at 37°C. *P < .05.
Flow cytometry analysis also revealed that IdeS-cleaved 5B9 could not inhibit the platelet binding of IV.3, a monoclonal Ab specific to FcγRIIA, while a dose-dependent inhibition of this binding was evidenced with intact 5B9 (Figure 1D). Moreover, functional assays performed with sc5B9 fragments obtained after incubating 5B9 with IdeS showed that unlike intact 5B9, sc5B9 was unable to induce platelet activation or aggregation in the presence of low concentrations of UFH (Figure 1E-F).
Cleavage in WB of anti-PF4/H Abs by IdeS prevents heparin-dependent platelet aggregation
The effect of IdeS was further studied in WB containing 5B9 or human HIT Abs. After 6 minutes of incubation with IdeS, the plasma obtained after WB centrifugation was incubated in micro wells containing PF4/H complexes, and the IgG fractions obtained after elution were examined by western blot analysis. These experiments clearly confirmed the full and dose-dependent cleavage of 5B9 by IdeS in WB. Intact IgG was almost undetectable when WB was incubated with the highest concentration of IdeS (ie, 0.02 U/µg IgG) (Figure 2A). IdeS was also efficient in generating scIgG Abs to PF4/H in WB containing HIT plasma samples (Figure 2D). This cleavage was not specific and also affected all other IgG (supplemental Figure 1A,C, available on the Blood Web site). Importantly, the IdeS cleavage of 5B9 or human HIT IgG did not affect the ability of Abs to bind PF4/H complexes, as confirmed by ELISA. Indeed, similar absorbance values were measured with samples treated and untreated by IdeS (supplemental Figure 1B,D). In addition, IdeS incubated at 0.005 and 0.02 U/µg IgG for 6 minutes in WB containing 5B9 fully abolished heparin-dependent platelet aggregation (Figure 2B). In contrast and as expected, platelet aggregation induced by collagen (0.5 µg/mL) or ADP (10 µM) was not affected by IdeS (Figure 2C). IdeS also inhibited heparin-dependent platelet aggregation when incubated for 6 minutes in WB containing HIT plasma samples (Figure 2E). In addition, IdeS fully abolished the serotonin release induced by these HIT samples in the presence of 0.1 IU/mL heparin, but platelet activation by thrombin remained unmodified (Figure 2F).
Cleavage of anti-PF4/H IgG by IdeS in WB and impact on H-dependent platelet activation and aggregation. (A,D) Western blot analysis after cleavage in whole blood by IdeS of 5B9 (A) or human HIT IgG (D) (n = 2 experiments). The amount of IdeS added varied from 0.002 to 0.02 U/μg IgG, considering that IgG concentration in whole blood from healthy donors was ∼7.5 mg/mL. (B,C-E) Representative platelet aggregation curves obtained with 5B9 (B), collagen and ADP (C), and human HIT plasma samples (E), without and with IdeS (n = 3 experiments). (F) Maximal serotonin release (mean ± SEM; n = 3 platelet donors) induced by thrombin (1 U/mL), 5B9 (20 µg/mL), or HIT plasma samples with 0.1 IU/mL UFH, previously treated or not with IdeS (0.02 U/µg IgG; 6 minutes at 37°C). All experiments were performed with whole blood from 2 or 3 healthy donors. *P < .05. AU, arbitrary units.
Cleavage of anti-PF4/H IgG by IdeS in WB and impact on H-dependent platelet activation and aggregation. (A,D) Western blot analysis after cleavage in whole blood by IdeS of 5B9 (A) or human HIT IgG (D) (n = 2 experiments). The amount of IdeS added varied from 0.002 to 0.02 U/μg IgG, considering that IgG concentration in whole blood from healthy donors was ∼7.5 mg/mL. (B,C-E) Representative platelet aggregation curves obtained with 5B9 (B), collagen and ADP (C), and human HIT plasma samples (E), without and with IdeS (n = 3 experiments). (F) Maximal serotonin release (mean ± SEM; n = 3 platelet donors) induced by thrombin (1 U/mL), 5B9 (20 µg/mL), or HIT plasma samples with 0.1 IU/mL UFH, previously treated or not with IdeS (0.02 U/µg IgG; 6 minutes at 37°C). All experiments were performed with whole blood from 2 or 3 healthy donors. *P < .05. AU, arbitrary units.
IdeS treatment inhibits in vitro thrombus formation and TF synthesis induced by 5B9 and H
WB from healthy donors preincubated with 5B9 was perfused (500 s−1) over a surface coated with human vWF 30 minutes after the addition of UFH. In this condition, many large platelet/leukocyte aggregates with fibrin(ogen), which covered ∼50% of coated surfaces, were observed after 5 minutes of WB perfusion, whereas these aggregates were very small or absent when WB without 5B9 was perfused with only UFH (Figure 3A-B). In addition, the presence of fibrin in clots induced by 5B9 and heparin was confirmed by using a specific antifibrin Ab, supporting a full activation of coagulation cascade (Figure 3C). In contrast, when IdeS was added in WB containing 5B9 (6 minutes; 0.02 U/ µg of IgG), the formation of large platelet/leukocyte aggregates and fibrin after addition of heparin was fully abolished (Figure 3A), with a strong and significant decrease in covered surfaces (Figure 3B).
IdeS inhibits thrombus formation and TF synthesis induced by 5B9 and UFH. (A) Representative images corresponding to areas of 0.1 mm2 vWF-coated microfluidic channels perfused (5 minutes, 500 s−1; 20 dyn/cm2) with recalcified WB incubated for 30 minutes in the presence of UFH (0.5 IU/mL) without or with 5B9 alone (40 µg/mL) or 5B9 + IdeS (0.02 U/µg of IgG). In experiments with IdeS, WB containing 5B9 was preincubated with IdeS for 6 minutes at 37°C before the addition of UFH. Platelets are shown in green (DiOC6), fibrin(ogen) is in red (Alexa Fluor 647–labeled fibrinogen), and leukocytes are blue (Hoechst 33342, DNA dye). Experiments were performed with WB from 2 healthy donors. (B) Percentages of area covered by platelet/leukocyte aggregates and fibrin(ogen) after infusion of UFH, UFH + 5B9 without or with IdeS. (C) Representative microscopy images of a clot observed after perfusion (5 minutes, 500 s−1) with recalcified WB incubated for 30 minutes with 5B9 (40 µg/mL) and UFH (0.5 IU/mL). Platelets are shown in green (DiOC6), leukocytes are blue (Hoechst), and fibrin was visualized (purple staining) after adding a Cy3-labeled antifibrin specific monoclonal Ab (clone 59D8) (5 µg/mL). Images were acquired using a ORCA-R2 digital CCD camera with a 20× objective; scale bars, 20 µm. (D) Relative TF mRNA synthesis (mean ± SEM) after the addition of 5B9 (50 µg/mL) or LPS (1 µg/mL) to WB and incubated without or with IdeS (0.02 U/µg IgG; 6 minutes at 37°C) before the addition of UFH (0.5 IU/mL). *P < .05; NS, not significant.
IdeS inhibits thrombus formation and TF synthesis induced by 5B9 and UFH. (A) Representative images corresponding to areas of 0.1 mm2 vWF-coated microfluidic channels perfused (5 minutes, 500 s−1; 20 dyn/cm2) with recalcified WB incubated for 30 minutes in the presence of UFH (0.5 IU/mL) without or with 5B9 alone (40 µg/mL) or 5B9 + IdeS (0.02 U/µg of IgG). In experiments with IdeS, WB containing 5B9 was preincubated with IdeS for 6 minutes at 37°C before the addition of UFH. Platelets are shown in green (DiOC6), fibrin(ogen) is in red (Alexa Fluor 647–labeled fibrinogen), and leukocytes are blue (Hoechst 33342, DNA dye). Experiments were performed with WB from 2 healthy donors. (B) Percentages of area covered by platelet/leukocyte aggregates and fibrin(ogen) after infusion of UFH, UFH + 5B9 without or with IdeS. (C) Representative microscopy images of a clot observed after perfusion (5 minutes, 500 s−1) with recalcified WB incubated for 30 minutes with 5B9 (40 µg/mL) and UFH (0.5 IU/mL). Platelets are shown in green (DiOC6), leukocytes are blue (Hoechst), and fibrin was visualized (purple staining) after adding a Cy3-labeled antifibrin specific monoclonal Ab (clone 59D8) (5 µg/mL). Images were acquired using a ORCA-R2 digital CCD camera with a 20× objective; scale bars, 20 µm. (D) Relative TF mRNA synthesis (mean ± SEM) after the addition of 5B9 (50 µg/mL) or LPS (1 µg/mL) to WB and incubated without or with IdeS (0.02 U/µg IgG; 6 minutes at 37°C) before the addition of UFH (0.5 IU/mL). *P < .05; NS, not significant.
A significant increase in TF mRNA levels was also evidenced when 5B9 was incubated in WB with UFH compared with the experimental condition without 5B9 (mean increase in TF mRNA level = 8.6-fold vs 1-fold; Figure 3D), but this increase in the TF mRNA level was no longer evident (mean = 0.9-fold ie, similar to those measured in non-stimulated condition) when WB with 5B9 was incubated with IdeS (0.02 U/µg IgG). Comparatively, IdeS treatment did not affect TF mRNA synthesis induced by LPS (mean increase in TF mRNA level: 17-fold vs 26-fold with or without IdeS, respectively).
IdeS prevents thrombocytopenia and thrombin generation induced by 5B9 in the hFcγRIIA/hPF4 transgenic mouse model
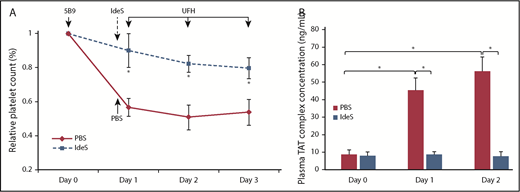
When 5B9 was injected with UFH in transgenic mice expressing human FcγRIIA and human PF4, significant thrombocytopenia occurred, with a 43% to 49% decrease in platelet count between days 1 and 3, respectively, as compared with the basal value (Figure 4A). Simultaneously, plasma levels of TAT dramatically increased on days 1 and 2 after injection with 5B9 and UFH as compared with day 0 (mean: 45 and 56 ng/mL vs 8.7 ng/mL; Figure 4B). However, when IdeS was injected in 5B9-sensitized mice 2 hours before UFH administration, the platelet count did not significantly decrease (ranging from 10% on day 1 to 20% on day 3), and TAT levels remained unchanged (mean: 8.6 and 7.7 ng/mL on days 1 and 2 vs 8.1 ng/mL on day 0; Figure 4A-B).
IdeS prevents thrombocytopenia and thrombin generation induced by 5B9 in vivo. (A) Relative decrease in platelet count compared with day 0 after injection of UFH in transgenic 5B9-treated mice (hFcγRIIA, hPF4 transgenic, mPF4 knockout) having received IdeS (25 U/g; n = 3) or PBS (n = 3). (B) TAT levels on days 0, 1, and 2. Data are mean ± SEM. *P < .05.
IdeS prevents thrombocytopenia and thrombin generation induced by 5B9 in vivo. (A) Relative decrease in platelet count compared with day 0 after injection of UFH in transgenic 5B9-treated mice (hFcγRIIA, hPF4 transgenic, mPF4 knockout) having received IdeS (25 U/g; n = 3) or PBS (n = 3). (B) TAT levels on days 0, 1, and 2. Data are mean ± SEM. *P < .05.
Discussion
The binding of anti-PF4/H IgG to Fcγ receptors, especially FcγRIIA, induces strong platelet and monocyte activation, which is critical in the pathogenesis of HIT and associated thrombotic complications.6,7 In this study, we demonstrated that IdeS cleavage of anti-PF4/H IgG Abs abolished their ability to activate platelets and monocytes and their pathogenicity.
IdeS is a cysteine protease isolated from S pyogenes, and several studies showed that it specifically cleaves IgG, without any other substrate identified.30,31 In our study, we demonstrated that IdeS cleaved 5B9, a recently developed chimeric anti-PF4/H Ab with a human IgG1 heavy chain comprising CH1, hinge, CH2, and CH3 domains.23 The cleavage of the first heavy chain of 5B9 was very fast and led to sc5B9 after only 3 minutes of incubation at 37°C, whereas that of the second heavy chain occurred after a longer delay and was completed only after overnight incubation. This result agrees with a previous study showing that the generation of scIgG was 40- to 100-fold faster than that of F(ab′)2.32
As expected, the cleavage of 5B9 into sc5B9 by IdeS did not alter its ability to bind PF4/H complexes. However, sc5B9 failed to activate platelets, and this effect was likely due to its inability to bind platelet FcγRIIA, as strongly supported by flow cytometry. IdeS cleaves the IgG1 lower hinge region between glycine residues 236 and 237 within the sequence PELLGGPSV,16,31 which is critical in the interaction of IgG with FcγRs.12 IdeS cleavage of anti-PF4/H Abs was also efficient in WB, with all IgGs degraded, as previously demonstrated ex vivo and in vivo.19-21 Like 5B9, human HIT Abs are predominantly IgG1, but some are IgG2 and IgG3,33 which are also cleavable by IdeS, although less efficiently for IgG2.30,31
Anti-PF4/H Abs cleaved by IdeS in WB were unable to activate platelets in the presence of UFH, and this effect was highly specific, because the platelet response to collagen or ADP was fully preserved. Moreover, experiments with transgenic mice expressing human FcγRIIA and PF4 demonstrated that IdeS administration 2 hours before UFH injection efficiently prevented the occurrence of severe thrombocytopenia induced by 5B9, and this effect was likely related to IgG cleavage by IdeS. Only a minor decrease in platelet count was observed in IdeS-treated mice, likely resulting from the UFH injection itself or from hemodilution secondary to frequent blood samplings in mice, as proposed elsewhere.28
IdeS was previously demonstrated to prevent depletion of platelets and the development of hemorrhagic purpura in a mouse model of immune thrombocytopenia.20 In addition, IdeS has also been used to prevent IgG-dependent glomerulonephritis or arthritis in different mouse models.34,35 In these studies, a unique dose of IdeS ranging from 5 to 25 µg/g was injected, but we administered a lower dose, 0.5 µg/g (25 U/g), to our transgenic mice. Of note, a similar dose of IdeS (0.24 to 0.5 mg/kg) was successful in preventing graft rejection in patients when injected 4 to 6 hours before the transplantation of a kidney from an HLA-incompatible donor.21
The thrombin-antithrombin level, previously validated as a surrogate marker of thrombosis in patients with HIT,36 was significantly increased in mice that had received 5B9 and UFH. However, IdeS injection 2 hours before H administration totally suppressed this increase in TAT levels in 5B9-treated mice. Thrombotic complications in HIT result from multicellular activation that involves platelets, monocytes, neutrophils, and endothelial cells,5,37-40 with an important role for TF and procoagulant microparticles in triggering and amplifying the coagulation cascade.6,7,41-44 In this regard, IdeS fully abolished monocyte TF mRNA synthesis induced in WB by 5B9 in the presence of H, with no effect on TF expression induced by LPS. Besides FcγRIIA, FcγRI could also play a role in monocyte activation induced by HIT Abs,43 and IdeS cleavage of anti-PF4/H IgG Abs also likely inhibits their binding to FcγRI and subsequent cellular activation.16 Finally, we demonstrated with a microfluidic model that a 6-minute pretreatment of 5B9-containing WB with IdeS was sufficient to completely inhibit heparin-dependent formation of fibrin/platelet clots. These results suggest that heparin could be administered in patients for a short window period after treatment by IdeS without increasing the risk of thrombotic events.
HIT is a severe clinical syndrome, and life-threatening thrombosis may develop in some patients. In addition, HIT patients may require emergency cardiac surgery with cardiopulmonary bypass (CPB), and UFH is the safest and easiest anticoagulant for use during CPB.45 Preoperative plasmapheresis has been proposed to strongly reduce the plasma level of PF4/H Abs, then allowing H use for anticoagulation during CPB without severe adverse events.46 However, plasma exchange is associated with potential harms, including procedural risk and complications due to the use of donor plasma. High-dose IV immunoglobulin has also been proposed in treating some patients with platelet activating-Abs and refractory HIT, but few experiences have been reported in the cardiac surgery setting.47-49 Therefore, IdeS injection could be a useful treatment of patients in whom pathogenic HIT Abs need to be rapidly inactivated. In cardiac surgery patients, IdeS could be administered just before CPB and UFH injection, because it is rapidly efficient with a short half-life of ∼4.9 (±2.8) hours.19 A rebound phenomenon with increased anti-PF4/polyanion Ab titers after UFH reexposure is likely rare and not a significant concern in clinical practice, when platelet-activating HIT Abs are already present in the patient blood. Indeed, in this case, the main objective is to inhibit the pathogenic effect of HIT Abs, which is why the most recent American Society of Hematology guidelines have also proposed the use of “intraoperative H in combination with a potent antiplatelet agent (eg, prostacyclin or tirofiban analogue)” when delaying heart surgery is not feasible.22
On the other hand, after IV injection of IdeS, nearly all IgG is cleaved and nonfunctional, and this might expose treated patients to an increased risk of bacterial infections. Therefore, polyclonal IgG infusion could be proposed early in the postoperative period to restore normal IgG levels in cardiac surgery patients who received IdeS before CPB. No clinically severe adverse events have been reported in healthy donors and renal transplant recipients who received IdeS.19,21,50 In addition, this protease does not appear to affect blood coagulation, with no impact on thrombin generation (data not shown). Noticeably, anti-IdeS Abs have been detected in most healthy donors in several studies, probably due to previous pyogenic streptococcal infections. These Abs did not have an impact on the effect of IdeS in treated subjects, suggesting that screening for such Abs in patients is not necessary in most cases before injection of IdeS.19,21,50 Moreover, a secondary immune response with increased levels of antiprotease Abs 2 to 3 weeks after infusion has also been demonstrated,50 possibly restricting subsequent and close administrations of IdeS. However, it has been also shown that after 6 to 12 months, anti-IdeS Ab levels return to baseline.
In conclusion, our study demonstrates that IdeS prevents in vitro and in vivo heparin-dependent cellular activation induced by HIT IgG Abs. IdeS fully abolished the pathogenicity of anti-PF4/H IgG Abs. This protease could be considered as a potential treatment for patients with severe HIT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Institut pour la Recherche sur la Thrombose et l’Hémostase, the Fond de dotation CSL Behring, and the program “Investissements d'Avenir” (grant agreement no. LabEx MAbImprove ANR-10-LABX-53-01).
Authorship
Contribution: C.K.-M. and J.R. performed and designed the research, analyzed the data, and wrote the paper; Y.G. designed the research, analyzed the data, and wrote the paper; Q.D., Y.Z., and S.L. performed research, analyzed the data, and wrote the paper; C.V. performed research and analyzed the data; and G.T., S.E.M., and C.P. designed the research and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yves Gruel, Department of Hematology-Hemostasis, CHU Tours, 37044 Tours Cedex, France; e-mail: gruel@med.univ-tours.fr.


![Figure 1. Cleavage of purified 5B9 by IdeS. Representative Coomassie blue SDS-PAGE (n = 3 experiments) (A) and percentages of intact 5B9 IgG, single cleaved 5B9 (sc5B9), and F(ab′)2 5B9 (mean values, n = 3), as defined by ImageJ, after incubation of 5B9 with IdeS (0.02 U/µg IgG) at 37°C (B). (C) Binding of cleaved 5B9 (1 µg/mL) to PF4/H complexes, as evaluated by ELISA with a secondary Ab specific to murine K chain (mean ± standard error of the mean [SEM], n = 2 experiments). A, absorbance; O/N, overnight. (D) Inhibition of IV.3 binding on platelets with increasing concentrations of 5B9 or sc5B9 (mean ± SEM; n = 3 platelet donors). (E) Representative platelet aggregation curve (n = 2 experiments) with untreated 5B9 (50, 25, 12.5, and 6.25 µg/mL) or sc5B9 (50 µg/mL) added to platelet-rich plasma with UFH (0.5 IU/mL). (F) Representative profile of serotonin release assay (n = 2 experiments) with untreated 5B9 (20 µg/mL) and sc5B9 (20 µg/mL) and different concentrations of UFH. (D-F) Single cleaved 5B9 (sc5B9) is the IgG fraction obtained after incubating 5B9 with IdeS (0.02 U/µg IgG; 6 minutes) at 37°C. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/22/10.1182_blood.2019000437/3/m_bloodbld2019000437f1.png?Expires=1769202670&Signature=PlHHq7AN1k7FGAVEuWHMkEhtOSRCBV1BU7ZEc~mjY7TeHjFyTNTmOeOLbXoRQgFYyKVQEZxln1JaZxGLx8RIV2ekqbGwFtUZEJScQYhFciPPMDcCFCsIYqLrC694cT3cZMIr3GfcRp-g0ZimNXBfQrdsblwr8BoCEbwXKECoNQmlO1H1ook~XthSsLgDRjJuBJYyYDrDu~oE1apaW20joYDVdcV5C3iDVJBcM-sQ2xD2x089N-YSKdyjOoQTGF5nWu~Mzt1x4PgA2SWAHZAYDGfl6wTKNiyvkFID8mYivCqrd8nSBFxW6oPfreobOBk7nqmaTe00oNeyOrFTGsZJwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 1. Cleavage of purified 5B9 by IdeS. Representative Coomassie blue SDS-PAGE (n = 3 experiments) (A) and percentages of intact 5B9 IgG, single cleaved 5B9 (sc5B9), and F(ab′)2 5B9 (mean values, n = 3), as defined by ImageJ, after incubation of 5B9 with IdeS (0.02 U/µg IgG) at 37°C (B). (C) Binding of cleaved 5B9 (1 µg/mL) to PF4/H complexes, as evaluated by ELISA with a secondary Ab specific to murine K chain (mean ± standard error of the mean [SEM], n = 2 experiments). A, absorbance; O/N, overnight. (D) Inhibition of IV.3 binding on platelets with increasing concentrations of 5B9 or sc5B9 (mean ± SEM; n = 3 platelet donors). (E) Representative platelet aggregation curve (n = 2 experiments) with untreated 5B9 (50, 25, 12.5, and 6.25 µg/mL) or sc5B9 (50 µg/mL) added to platelet-rich plasma with UFH (0.5 IU/mL). (F) Representative profile of serotonin release assay (n = 2 experiments) with untreated 5B9 (20 µg/mL) and sc5B9 (20 µg/mL) and different concentrations of UFH. (D-F) Single cleaved 5B9 (sc5B9) is the IgG fraction obtained after incubating 5B9 with IdeS (0.02 U/µg IgG; 6 minutes) at 37°C. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/22/10.1182_blood.2019000437/3/m_bloodbld2019000437f1.png?Expires=1770695790&Signature=S3rodniDz3nLLNwYgkgAt2ZmvVyqEk24E1qDQjwZlxd-ZdP-aWZAPL~jZX74EuZ3lSwKbzzsV46lgqDJnO1JNp-LtiR5zUK41Qn7JCzvRUX0R2YPlw1sMrnxx2OMPjnGKHw~t8vXgZl~AH6xnOcxS7LGCSBordXtgSu9qDnfsW3EBWL6kW~JHYDn53koWumaoGJeVQc-HZcuft6DDKH533Y3uBAoKQzXYCdm-ekYB5eWyC5t~QOTuo5qxe0KC84~CMNMdsya7uCWCaQUT3VnaduoRqGx5uLj2maRJup6rL1QluoaZzOU0efzqWv2zk8~zYDNld0r9V5Vvj1~wcQ-Ug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)