Abstract
Biologic agents are among the fastest-growing classes of therapeutic products. A biosimilar therapeutic product is defined as one that is similar to an already licensed reference product in regard to quality, safety, and efficacy and is often priced more competitively (Expert Committee on Biological Standardization, 2009; Publicover, et al., 2013).
In 2016, the Saskatchewan Cancer Agency effectively changed from a granulocyte colony-stimulating factor (G-CSF) brand name product (Neupogen®) to a biosimilar (Grastofil®) for stem cell mobilization prior to autologous stem cell transplants (ASCT). However, because its efficacy in this setting is currently unknown, many institutions continue to use the brand name product. To address this, we reviewed patient charts and compared the efficacy of CD34+ collection in 170 patients who received Neupogen® and 47 patients who received Grastofil® between 2012 and 2018.
Additionally, we analyzed efficacy of mobilization with both G-CSF products either alone or in combination with chemotherapy, patients requiring more than one apheresis day and requirement for Plerixafor®. Time to engraftment, and length of stay in hospital post ASCT were used as clinical efficacy parameters. This analysis is important to ensure that patient outcome is not compromised upon use of Grastofil® as opposed to the already approved reference, Neupogen®. The increased use of biosimilars would allow for decreased costs and more sustainable healthcare.
Results
Neupogen® and Grastofil® had similar efficacy for stem cell mobilization as 92.4% of the patients harvested with the brand name had a successful harvest compared to 100% of the patients given the biosimilar. A successful harvest is defined as a collection of ≥2x106 CD34+ cells for patients planned for one stem cell transplant and ≥4x106 CD34+ cells for patients planned for two transplants. Additionally, the two drugs did not display a statistically significant difference in Plerixafor requirement in a situation with low CD34+ count. Amongst all 217 patients, 25.9% of patients given Neupogen® required Plerixafor® as compared to 23.4% of Grastofil® patients.
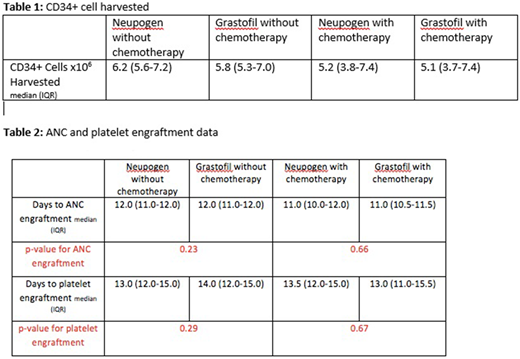
As seen from Table 1, by using the Wilcoxon test to compare the efficacy of Neupogen® and Grastofil® without chemotherapy for stem cell mobilization, there was no significant difference seen with a p-value of 0.53. Similarly, in patients mobilized with chemotherapy in addition to either Neupogen® or Grastofil®, similar efficacy was seen between the groups given a p-value of 0.95.
There was no statistically significant difference between the patient groups with respect to requiring more than 1 day of apheresis. 59.4% of patients mobilized with Neupogen® required more than 1 apheresis day compared to 76.9% of Grastofil® patients (p = 0.11). Similarly, of the Neupogen® and Grastofil® groups mobilized with chemotherapy, 42.5% and 38.1%, respectively, required more than one apheresis day which was not statistically different (p = 0.71). Table 2 presents the engraftment data which also suggests that the two drugs behave with similar efficacy in this respect.
Length of stay in hospital post autologous stem cell transplant was an additional variable analyzed. Again, there was no significant difference in length of stay between patients who received Neupogen® (median=18.5 days, IQR=17.0-21.0) or Grastofil® (median=19.0 days, IQR =17.0-22.0) without chemotherapy (p = 0.75). When chemotherapy was added to the mobilizing regimen, lengths of stay post autologous stem cell transplant increased but there was no statistically significant difference in length of stay between Neupogen® plus chemotherapy mobilization (median=22.0, IQR =20.0-26.0) versus Grastofil® plus chemotherapy (median=24.0 days, IQR =21.0-29.0) mobilization (p-value =0.10).
In conclusion, when comparing the use of either a Neupogen® or Grastofil® based mobilization regimen in terms of stem cell harvest success, Plerixafor® use, more than one apheresis day required, time to engraftment, and length of stay in hospital, no significant difference was found indicating that both products, the reference agent and its biosimilar have similar efficacy. The use of such biosimilars can provide substantial cost savings to the health care system.
Elemary:Roche: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Lundbeck: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.