Abstract
Introduction
Neoplasms of different lineages that arise in the same patient and show a clonal relationship are exceedingly rare, but have been described in instances of histiocytic and myeloid/lymphoid neoplasms (Durham BH, et al. Blood 2017;130(2):176-80; Ansari J, et al. Eur J Haematol 2016;97(1):9-16). The clonal relationships can be demonstrated by shared genetic abnormalities such as translocations, somatic mutations, and other chromosomal level alterations. Nevertheless, a clonal link between germ cell tumors (GCT), a group of solid tumors derived from primitive stem cells, and hematologic malignancies has been less well-characterized. Relevant literature has mostly reported associations of GCT with acute leukemias, without a comprehensive assessment of genetic alterations (Mukherjee S et al. Ann Hematol 2017;96: 1435-39). To further characterize this phenomenon, we identified a small cohort of patients diagnosed with both GCT and any myeloid/histiocytic neoplasm. We evaluated their molecular and cytogenetic alterations, identifying shared and unique abnormalities, providing evidence of a clonal relationship between these two groups of neoplasms, which traditionally represent different cellular origins.
Methods
A search of the pathology database at a major referral center (Memorial Sloan-Kettering Cancer Center) was performed to identify patients diagnosed with GCT between 2012-2018, and had at least 1 prior or subsequent bone marrow biopsy. The medical records were reviewed for details of clinical presentations and evidence of myeloid neoplasm, with corresponding morphologic, cytogenetic, and molecular findings. The findings were correlated with the genetic alterations detected in the GCT during diagnostic work-up. Cytogenetic analyses include karyotyping, fluorescence in situ hybridization (FISH) studies for common abnormalities among myeloid neoplasms, and single nucleotide polymorphism (SNP) array for copy number gain/loss and copy neutral-loss of heterozygosity (CN-LOH). Molecular analyses include an amplicon capture-based next generation sequencing (NGS) assay for 49 genes relevant for hematologic malignancies, and MSK-IMPACTTM, a hybrid capture-based NGS assay for mutation and copy number alteration in 400+ genes. Somatic nature of the identified variants was confirmed on MSK-IMPACTTM by germline variant filtering with the aid of appropriate normal control samples (blood or nail).
Results
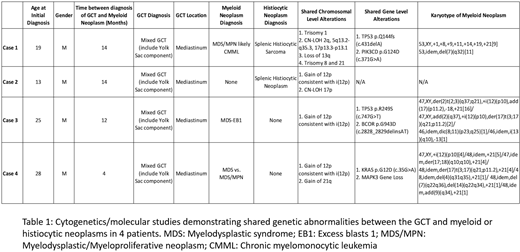
8 patients with GCT diagnoses showed marrow findings consistent with or suspicious for involvement by myeloid/histiocytic neoplasms, with clinical presentations not typical for therapy-related myeloid neoplasms. The patients were predominantly male (n=6) and young (age range: 13-55, median=26.5). The GCT included mixed GCT (n=6), mature teratoma (n=1) and immature teratoma (n=1). The primary sites of involvement were mediastinum (n=4), testes (n=2), and ovaries (n=2). In all cases, the myeloid/histiocytic neoplasms and GCT were diagnosed in close proximity in time (range: <1 to 24 months). So far, cytogenetic and molecular data in 4 patients with mediastinal-based tumors showed shared alterations between the GCT and myeloid/histiocytic neoplasms, including isochromosome 12p in 3 patients, an alteration characteristic of GCT. Other shared alterations observed included identical mutations in TP53, PIK3CD, KRAS, BCOR; trisomy 1, 8, 21; gain of 21q; loss of 13q; and CN-LOH of 2q, 5q, 17p. Interestingly, in all 4 patients, the GCT and myeloid/histiocytic neoplasm each showed additional unique genetic alterations.
Conclusion
These findings suggest that a subset of patients with GCT can develop clonally-related myeloid/histiocytic neoplasms, with a relatively short latency period. The genetic changes seen in our cases are relatively less common in conventional myeloid neoplasms, suggesting unique molecular pathogenesis. Yolk sac component in the GCT, which has been described to harbor hematopoietic precursor cells, may contribute to pathogenesis (Orazi A, et al Cancer 1993;71(12):3873-81). Although the detailed pathogenic mechanism remains uncertain, we observed that in the cohort, the GCT and myeloid/histiocytic neoplasm each harbored additional unique genetic changes. This suggests the presence of a common neoplastic progenitor giving rise to neoplasms of different lineages, which subsequently accumulated additional alterations.
Ho:Invivoscribe, Inc.: Honoraria. Yabe:Y-mAbs Therapeutics: Consultancy. Arcila:Invivoscribe, Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.