Abstract
In 2017, the Food and Drug Administration approved 2 medications for sickle cell anemia (SCA): hydroxyurea for children and l-glutamine for children and adults. The approval of hydroxyurea was long overdue, but the approval of l-glutamine was a surprise to many. Any effective new treatment for SCA is a welcome advance, but there are few published studies of l-glutamine as a specific treatment for SCA. Accordingly, there are many unanswered questions about its efficacy, safety, and role in current therapy.
Glutamine
Glutamine is one of the most abundant amino acids in humans and a vital oxidative fuel for rapidly proliferating human tissues.1,2 Glutamine is involved in nitrogen transport, regulation of acid-base homeostasis, and catabolic signaling. It is a gluconeogenic substrate in certain tissues, and it is required for the synthesis of other amino acids, proteins, nucleic acids, nucleotides, and hexosamines. Of particular interest to hematologists, glutamine is a precursor for the synthesis of glutathione (GSH), nicotinamide adenine dinucleotide (NAD), and arginine, all of which protect erythrocytes from oxidative damage and indirectly maintain vascular tone. However, when interpreting the clinical trials of l-glutamine in SCA, it is important to consider all the roles of glutamine in the body, not just its effects in erythrocytes.
Early, preclinical studies of l-glutamine for SCA
l-Glutamine has been investigated as a treatment of SCA for >40 years. In the 1970s, there was great interest in finding nontoxic “antisickling” agents following initial studies that identified urea, cyanate, carbamoyl phosphate, and other compounds with promising activity. In 1975, Rumen published an article in Blood entitled, “Inhibition of sickling in erythrocytes by amino acids.”3 He examined deoxygenated hemoglobin (Hb) S-containing erythrocytes on microscope slides under sealed coverslips and found that erythrocytes in suspensions of either l-asparagine, l-glutamine, or l-homoserine at 3.8 mM had at least 90% fewer sickled forms than those in saline suspensions. Rumen included photomicrographs of the apparent antisickling properties of these amino acids, and he concluded that, although the mechanism of action was unknown, “…it might be possible to prevent sickle cell crises by dietary means.”3
In 1976, Mackenzie et al developed a novel apparatus to screen compounds for antisickling properties in whole blood.4 They found that l-glutamine at 3.8 mM had no measurable effect on the rate of erythrocyte sickling.4 They speculated that l-glutamine had no effect because it was mostly bound to plasma proteins (Rumen used erythrocyte suspensions3 ), so less was available for interaction with Hb. Later, in 1980, Shirahama et al challenged the notion that the shape changes induced by l-glutamine in vitro necessarily indicated favorable “antisickling” effects.5 They found that neither l-glutamine, l-asparagine, nor l-homoserine had any effect on the minimum gelling concentration of deoxyhemoglobin S. Likewise, none of these amino acids (50 mM) affected the filterability of deoxygenated sickle erythrocytes through uniform sieves. Despite this, their own “…microscopic analysis of the ‘reversed’ sickle cells in the presence of three amino acids was essentially the same as that observed by Rumen.” Accordingly, they reasoned that these morphological changes could not be regarded as reversal of sickling and must be the result of other effects on the erythrocyte membrane (eg, changes in volume homeostasis). They concluded that the “…use of these compounds (eg, l-glutamine) as antisickling agents is therefore doubtful.”5
Erythrocytes and glutamine
In 1988, a group of investigators led by Kouichi Tanaka reported a decreased ratio of reduced NAD to total NAD in sickle erythrocytes compared with normal, driven entirely by an increase in total NAD.6 Given that glutamine is a precursor to NAD (Figure 1), this group went on to study erythrocyte glutamine biology. In 1997, Niihara et al reported markedly increased glutamine transport into sickle erythrocytes, compared with normal and high reticulocyte controls, resulting from increased glutamine affinity and maximal velocity of the Na-dependent glutamine transporter.7 The intracellular glutamine concentration of sickle erythrocytes was not increased, but glutamate was.7 Glutamate, which has no specific receptor-mediated transport across the erythrocyte membrane, is also a by-product of NAD synthesis derived from glutamine (Figure 1). This group theorized that increased NAD turnover due to ongoing oxidative injury, coupled with a compensatory increase in glutamine transport to support increased NAD synthesis, could explain their findings.7 These observations formed the basis for their subsequent studies of l-glutamine in SCA.
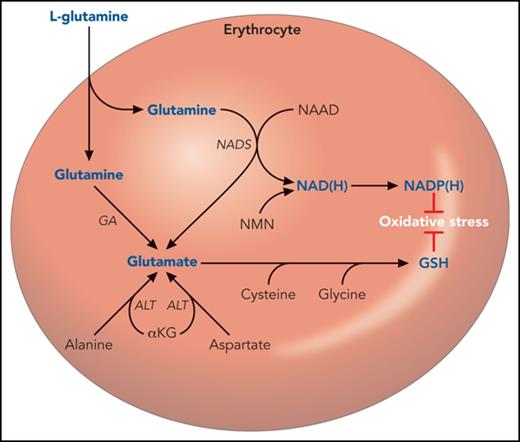
A hematologist’s narrow view of glutamine and glutamate metabolism. Glutamine is transported across the erythrocyte membrane, where it can support the synthesis of NAD(H), NADP(H), GSH, and glutamate. However, these can also be synthesized in pathways that do not require glutamine as a proximal precursor. For example, the majority of erythrocyte glutamate appears to derive from alanine via ALT.10 NAD(P) also derives from nicotinamide mononucleotide. NADP(H) and GSH are used by the erythrocyte to counter oxidative injury. Several intermediate steps are omitted for clarity. αKG, α-ketoglutarate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GA, glutamine aminohydrolase; NAAD, nicotinic acid adenine dinucleotide; NAD(H), reduced nicotinamide adenine dinucleotide; NADP(H), reduced nicotinamide adenine dinucleotide phosphate; NADS, nicotinamide adenine dinucleotide synthase; NMN, nicotinamide mononucleotide.
A hematologist’s narrow view of glutamine and glutamate metabolism. Glutamine is transported across the erythrocyte membrane, where it can support the synthesis of NAD(H), NADP(H), GSH, and glutamate. However, these can also be synthesized in pathways that do not require glutamine as a proximal precursor. For example, the majority of erythrocyte glutamate appears to derive from alanine via ALT.10 NAD(P) also derives from nicotinamide mononucleotide. NADP(H) and GSH are used by the erythrocyte to counter oxidative injury. Several intermediate steps are omitted for clarity. αKG, α-ketoglutarate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GA, glutamine aminohydrolase; NAAD, nicotinic acid adenine dinucleotide; NAD(H), reduced nicotinamide adenine dinucleotide; NADP(H), reduced nicotinamide adenine dinucleotide phosphate; NADS, nicotinamide adenine dinucleotide synthase; NMN, nicotinamide mononucleotide.
Studying other mechanisms of oxidative sensitivity in SCA, Kiessling et al reported that the low level of reduced GSH in sickle erythrocytes was not explained by decreased substrate availability (cysteine, glycine, and glutamate via glutamine).8 Instead, they found increased erythrocyte concentrations of all these amino acids, compared with controls, suggesting increased consumption of GSH due to oxidative stress rather the substrate-limited synthesis of GSH.8 These findings were corroborated, in part, by Morris et al, who showed a decreased glutamine-to-glutamate ratio in sickle erythrocytes, primarily driven by increased amounts of glutamate.9 Of note, plasma and erythrocyte glutamine levels were not different between SCA patients and controls, but a subgroup of SCA patients with a tricuspid regurgitant jet velocity ≥2.5 m/s had lower erythrocyte glutamine.9
Assuming that intracellular glutamine is the major source of glutamate for GSH synthesis in erythrocytes, some have postulated that pharmacological supplementation of l-glutamine in SCA could augment GSH synthesis. However, as discussed above, GSH synthesis is not substrate-limited. Furthermore, in 2001, Ellinger et al reported that ∼90% of erythrocyte glutamate is actually derived from α-ketoglutarate and alanine (catalyzed by alanine aminotransferase) rather than glutamine (Figure 1).10 To complicate the issue more, the measured concentrations of glutamine in sickle erythrocytes across these studies were disparate: normal in Niihara et al,7 increased in Kiessling et al,8 and decreased in Morris et al (in the tricuspid regurgitant jet velocity ≥2.5 m/s subgroup).9 Taken together, these reports do not provide a strong rationale for pharmacological l-glutamine supplementation to augment erythrocyte GSH synthesis.
l-Glutamine as a treatment for SCA
Based on their earlier work showing decreased NAD redox potential (NADH/[NAD+ + NADH]) and increased glutamine transport into sickle erythrocytes,6,7 Niihara et al administered 30 g/d of l-glutamine for 4 weeks to 7 individuals with SCA (Table 1).11 There were significant increases in NADH and NAD redox potential, but no change in Hb concentration. In a follow-up study, Niihara et al showed decreased in vitro endothelial adhesion of sickle erythrocytes from 11 individuals with SCA who were given a similar regimen of oral, high-dose l-glutamine.12 Again, there were no changes in Hb concentration or reticulocyte counts. Erythrocyte morphology was reported to have “improved,” but this should be interpreted in the context of the findings of Shirahama et al from 1980.5
A different group of investigators tested whether l-glutamine could reduce resting energy expenditure (REE) and improve growth in children with SCA (Table 1).13 This was based on several prior reports of increased REE, protein turnover, and glutamine utilization in SCA, as well as the potential benefits of l-glutamine in many other conditions.2 Williams et al reported in 2004 that 24 weeks of glutamine (0.3 g/kg twice daily) was associated with a 6% decrease in REE and increased plasma glutamine, but no change in total Hb concentration.13
The first controlled trial of l-glutamine for SCA was reported by Niihara et al in 2014 (Table 1).14 Eighty-one participants were randomized (1:1) to oral l-glutamine (0.3 g/kg twice daily to a maximum of 30 g/d) or placebo for 48 weeks. The primary outcome measure was the number of vaso-occlusive painful events through week 48. There were very high withdrawal rates for both treatment groups (49% for l-glutamine, 62% for placebo). The trial failed on its primary end point (P = .08). In secondary analyses, there was a nominally significant decrease in the number of hospitalizations in the l-glutamine group at week 24 (P = .04) but not week 48 (P = .07). There were no changes in Hb or reticulocyte counts, although these were not reported numerically. Improvement in erythrocyte morphology (in vivo) was also reported. l-Glutamine was reported to be well tolerated, although 1 patient in the l-glutamine arm died during the study due to multiorgan failure that was deemed by the investigators to be unrelated to study drug.14
In the only phase 3 trial, 230 individuals with SCA and ≥2 painful events in the preceding year were randomized (2:1) to l-glutamine or placebo for 48 weeks (Table 1). A stable dose of hydroxyurea was permitted at study entry. Recent transfusions, renal insufficiency, uncontrolled liver disease, pregnancy, and lactation were exclusionary. The primary outcome was the number of “sickle cell crises” (a composite of painful events, acute chest syndrome, acute splenic sequestration, and priapism), and the trial was completed in March 2014. The findings have been presented in at least 4 conference abstracts,15-18 but a manuscript with the full results has yet to be published. Data from these abstracts,15-18 the FDA application,19 and the package insert20 indicate that l-glutamine therapy was associated with a statistically significant reduction, compared with placebo, in the median number of sickle cell crises: 3 (minimum, maximum: 0, 15) vs 4 (0, 15), P = .006. Similarly, there were reductions in median number of hospitalizations for painful events (2 vs 3) and cumulative days in hospital (6.5 vs 11), as well as increased median time to first crisis (84 vs 54 days). There were 2 deaths in the l-glutamine group that were deemed by the investigators not to be related to study drug.20 Interpretation of the trial’s results is complicated by high dropout rates (36% l-glutamine arm; 24% placebo arm).21 Nevertheless, the FDA decided that l-glutamine had a favorable risk-benefit profile and approved it for use in SCA.21
Mechanism of action, dosing, and price
The Endari formulation of l-glutamine is now available for prescription. Its mechanism of action is unclear. In the Endari package insert, the clinical benefit is speculated to derive from decreased susceptibility of sickle erythrocytes to oxidative damage. However, none of the clinical trial data published to date show any improvements in Hb or reticulocyte count, so there seems to be no readily appreciable diminution of hemolysis. The multitude of other roles of glutamine in the body needs to be considered instead.
The recommended dose is weight-based and consistent with the prior clinical trials (Table 1). Endari is supplied in 5 g packets, so patients will need to mix 1 to 3 packets of powder in a beverage or food twice daily. It is taken by mouth and requires no therapeutic drug monitoring, and these are attractive characteristics for any medication. However, the experience of the sickle cell community with the Exjade formulation of deferasirox (tablets for oral suspension) suggests that a powder mixed in a beverage or a food might actually pose significant barriers to adherence. The lack of specific laboratory monitoring (eg, drug levels and efficacy measurements) will be appreciated by patients and families, but this reflects less on the convenience of l-glutamine and more on our ignorance about its risks and benefits. We do not know how l-glutamine works or what the optimal dose is for individuals with SCA.
Endari is estimated to cost >$3000 per month for adults and $1000 per month for children, which is 20 times more expensive than hydroxyurea.22 Whether insurance companies will pay for this expensive new therapy remains to seen, but the FDA indication for SCA will likely help in this regard. Of note, there are potentially less expensive pharmaceutical formulations of l-glutamine available for prescription, but without the specific indication for SCA. Over-the-counter formulations of glutamine should probably be avoided, largely because the purity of these nutritional supplements is unclear and less regulated.
Safety, efficacy, and place in current therapy
l-Glutamine, in the dose recommended, appears to be mostly well tolerated, but long-term safety data are lacking in SCA. The most common adverse events are constipation, nausea, vomiting, headache, and abdominal pain.20 Many trials of l-glutamine have shown apparent safety across a number of conditions,1 but not all. In particular, critically ill patients with multiorgan failure randomized to 0.35 g/kg of IV glutamine daily had higher mortality rates than those who did not receive it (the REDOXS Study),23 consistent, in aggregate, with other multicenter trials of glutamine for critically ill patients.24 Renal failure, in particular, and hepatic dysfunction may underlie this association.25,26 Of note, there were 3 deaths in the l-glutamine arms (but none in the placebo arms) of the 2 randomized trials in SCA that were not deemed by the investigators to be related to l-glutamine.14,20 Limited clinical information is published only for the individual in the phase 2 trial who died of “multiorgan failure unrelated to study medication.”14 Given these data, l-glutamine supplementation should be used cautiously, if at all, in SCA patients with hepatic or renal impairment, and certainly stopped in those who develop hepatic, renal, or multiorgan failure. Accordingly, serial clinical laboratory monitoring of hepatic and renal function should be strongly considered for patients treated with l-glutamine, but the appropriate frequency of such testing is unknown.
Currently, it is reasonable to consider the addition of l-glutamine to hydroxyurea therapy, but only after the optimization of hydroxyurea therapy and not at the risk of reducing adherence to hydroxyurea. A danger is that some patients will succumb to the naturalistic fallacy and choose to take l-glutamine instead of hydroxyurea. For the small number of individuals who tolerate hydroxyurea poorly, l-glutamine could be considered primary therapy. Its added role, if any, in chronic transfusion therapy is unknown. For any treatment, we would prefer >1 randomized phase 3 trial to inform practice, so additional studies are needed, especially to quantify, as the primary outcome measure, any added benefit of l-glutamine to optimized hydroxyurea therapy. These trials will also need to generate the necessary mechanistic and biochemical data that are missing from the phase 3 trial.
In summary, a newly approved treatment of patients with SCA is a welcome advancement. However, peer-reviewed publication of the primary data will be needed for l-glutamine to gain the acceptance of the academic hematology community. The clinical benefit of l-glutamine appears to be modest, and it is much more expensive than hydroxyurea, the only other approved medication for SCA. There are no published, long-term data about its use in this population, but l-glutamine appears to be reasonably well tolerated in the short term. However, therapeutic vigilance will be needed, because people with SCA are at risk of renal insufficiency, hepatic dysfunction, and multiorgan failure, all conditions associated with increased mortality in other trials of l-glutamine.
Authorship
Contribution: C.T.Q. conceived and wrote the manuscript.
Conflict-of-interest disclosure: C.T.Q. has received research funding from Amgen, Inc and Global Blood Therapeutics, Inc.
Correspondence: Charles T. Quinn, Division of Hematology, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 7015, Cincinnati, OH 45229; e-mail: charles.quinn@cchmc.org.