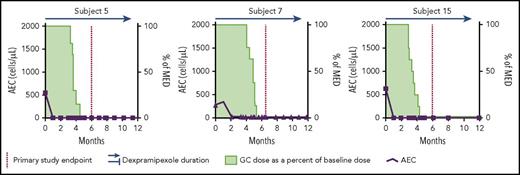
In this issue of Blood, Panch et al report that dexpramipexole reduces blood and tissue eosinophils and enables glucocorticoid reduction or cessation in patients with the hypereosinophilic syndromes (HESs).1 Four of the 10 treated patients met the primary end point. The figure shows findings in 3 responders, patients 5, 7, and 15, who had complete and sustained reductions in AECs. They discontinued glucocorticoids, and all were asymptomatic with AEC 0/μL beginning at about 2 months and persisting to a median of 29 months’ treatment. In patient 5, pre- and posttreatment biopsies of the esophagus and duodenum showed an absence of eosinophil infiltration at week 24. Adverse reactions did not lead to drug discontinuation. These results suggest that dexpramipexole is an effective treatment of some HES patients; however, only 3 of the 10 were impressive responders.
Patients with vigorous and sustained responses to dexpramipexole. Blood absolute eosinophil counts (AECs) are shown by bold line and squares (patients 5 and 15) and triangles (patient 7). MED is minimum effective glucocorticoid dose that maintained the AEC <1000/μL and suppressed symptoms of the hypereosinophilic syndrome (determined before drug administration). Green shading is the glucocorticoid dose as a % baseline or MED. The dotted vertical line indicates the primary study time end point. Dexpramipexole administration is indicated by the horizontal line/arrow above the graph. GC, glucocorticoid; MED, minimum effective glucocorticoid (prednisone or equivalent) dose.
Patients with vigorous and sustained responses to dexpramipexole. Blood absolute eosinophil counts (AECs) are shown by bold line and squares (patients 5 and 15) and triangles (patient 7). MED is minimum effective glucocorticoid dose that maintained the AEC <1000/μL and suppressed symptoms of the hypereosinophilic syndrome (determined before drug administration). Green shading is the glucocorticoid dose as a % baseline or MED. The dotted vertical line indicates the primary study time end point. Dexpramipexole administration is indicated by the horizontal line/arrow above the graph. GC, glucocorticoid; MED, minimum effective glucocorticoid (prednisone or equivalent) dose.
Although these results are very promising, the discovery of dexpramipexole as an eosinophil treatment is a remarkable story. Initially, dexpramipexole was proposed as a treatment of amyotrophic lateral sclerosis (ALS) based partly on enhanced brain mitochondrial function2 and the demonstration of increased survival rates and motor function retention in in vivo ALS models.3 However, an international clinical trial of almost 1000 ALS patients failed to show a difference from placebo in a combined assessment of function and survival,4 a discouraging result that might well have marked the end of its use. One effect that was more common in the dexpramipexole-treated group was a consistent reduction in blood eosinophils at dexpramipexole doses that were otherwise well tolerated.4,5 That finding stimulated 2 studies, 1 in HES patients and the other in patients with chronic rhinosinusitis with nasal polyps.6
The study of patients with chronic rhinosinusitis and nasal polyps (reported as an abstract)6 involved 11 subjects. After treatment with daily oral dexpramipexole 300 mg for 6 months, AECs were reduced almost 10-fold (P = .001), and in 7 of the 11 patients, AECs were reduced comparably to patients 5, 7, and 15 in the figure, an overall durable AEC reduction rate of 64%. Eosinophil counts per high power field in nasal biopsies from 10 patients changed from 190 to 11 (94% reduction from baseline, P = .004).
Although both studies were open label, the results in patients with durable and sustained responses are impressive and suggest that dexpramipexole could be an effective treatment of patients with eosinophil-related diseases. Because many asthma patients suffer from chronic rhinosinusitis with nasal polyposis and peripheral blood eosinophilia, one wonders if dexpramipexole might be of benefit to patients with eosinophilic asthma. Patients with myeloproliferative (primary, neoplastic) HES were excluded from the Panch et al study, but one also wonders if certain patients in whom a kinase target has not been identified would be helped.
The mechanism of dexpramipexole’s eosinophil-reductive activity is unknown. Bone marrow analyses in the HES patients showed mainly eosinophil precursors (promyelocytes), indicating the possibility of interference in an early step of eosinophil maturation. Presently, there is no in vitro assay to investigate dexpramipexole’s activity, and such a tool would be useful for studies to determine its mechanism of action and to probe related compounds that could be identified in a chemical library. Additionally, recognizing that a discouraging aspect of the reported study is that a robust response was observed only in 3 of the 10 HES patients; a test that identifies responders to the eosinophil-reductive effects would be valuable to determine which patients would benefit from the therapy and/or give clues as to how to make the therapy effective in more patients.
The take-home message is that dexpramipexole, a drug abandoned for lack of efficacy in its initial pharmacological application, shows promise as a well-tolerated orally administered therapy based on the serendipitous discovery of its ability to reduce eosinophils. In the 2 reports on HES and on chronic rhinosinusitis and nasal polyps, blood and tissue eosinophils were significantly diminished. These findings set the stage for phase 3 clinical trials in patients with common eosinophil-related diseases. Since the early 1950s, long-term glucocorticoid therapy, with its attendant adverse effects on most of the metabolic systems in the body, has been the mainstay of treatment of most eosinophil-related diseases. These early results encourage belief that this drug could herald a welcome change. As a final note, the patients responding to dexpramipexole appear devoid of eosinophils, raising consideration of whether this is a health hazard. However, review of patients (and mice) without eosinophils suggests that there are no obvious clinical consequences,7 at least in the absence of helminth infections.
Conflict-of-interest disclosure: The author declares no competing financial interests.