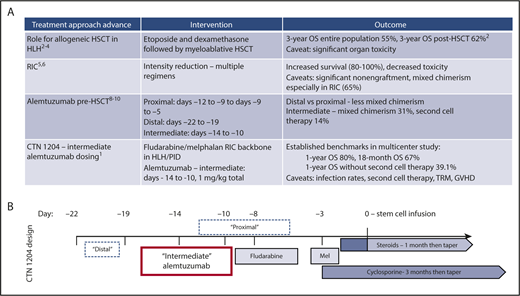
(A) Selected advances in role of stem cell transplantation in hemophagocytic lymphohistiocytosis and primary immunodeficiencies. (B) Schema of BMT CTN study 1204 transplant regimen and timing of alemtuzumab dosing (vs prior studies). Mel, melphalan; OS, overall survival; RIC, reduced-intensity conditioning; TRM, transplant-related mortality.
(A) Selected advances in role of stem cell transplantation in hemophagocytic lymphohistiocytosis and primary immunodeficiencies. (B) Schema of BMT CTN study 1204 transplant regimen and timing of alemtuzumab dosing (vs prior studies). Mel, melphalan; OS, overall survival; RIC, reduced-intensity conditioning; TRM, transplant-related mortality.
Large-scale global observational studies in pediatric HLH (familial, acquired, and associated with PIDs) established treatment algorithms containing etoposide and steroids and a curative role for hematopoietic stem cell transplantation (HSCT) in 1994. Specifically, the HLH-1994 study experience in 113 children demonstrated 3-year survival of 55%; in the 58% of patients who underwent HSCT, survival was 62%. This represented a huge advance in treating what was previously an almost universally fatal disease of immune activation and dysregulation.2 Ten-year follow-up from HLH-1994 (n = 249), data from the HLH-2004 study involving up-front calcineurin inhibition (n = 369), and results from smaller cohorts demonstrated 5-year survival rates of 54% to 61%; again, those who were transplanted (∼50%) had better survival of 50% to 70%.3,4 Up to 20% of subjects may survive without transplantation, but HSCT is considered a necessary intervention for most individuals, especially those with familial HLH (see figure panel A).
Although encouraging, these cohorts highlighted an early mortality rate of close to 30% following HSCT with primarily myeloablative conditioning. Causes of transplant-related mortality included liver toxicity (particularly veno-occlusive disease), viral infections, respiratory and other organ failure, and graft-versus-host disease (GVHD).3,4 Introduction of RIC to reduce complications boosted survival rates to 75% to 100%.5 One retrospective analysis demonstrated 3-year survival of 43% following myeloablative conditioning (n = 14) vs 92% following RIC (n = 26).6 The most common RIC regimens used fludarabine, melphalan, and either alemtuzumab or antithymocyte globulin.
Unfortunately, reduction of toxicities with RIC regimens came with increased incidence of mixed chimerism (eg, >5% residual recipient or <95% donor cells) following HSCT: 65% after RIC vs 18% after myeloablative conditioning in 1 study.6 There are no data to correlate a specific threshold of total leukocyte or T-cell chimerism with optimal survival. However, multiple studies have shown >20% to 30% chimerism of donor cells is associated with, although not necessary for, sustained remission of HLH.7 This led to recent practice patterns wherein >80% of patients with mixed chimerism receive a second transplant, CD34+ selected stem cell boost, and/or T-cell containing donor lymphocyte infusion. In some trials, the majority of patients received at least 1 additional cellular therapy after RIC HSCT.7
Alemtuzumab has historically been used during HSCT conditioning to achieve in vivo T-cell depletion of the graft and decrease GVHD. In HLH, alemtuzumab alone has been shown to be an effective immunomodulatory salvage therapy in those failing etoposide and steroids.8 Therefore, utilization of alemtuzumab with HSCT for HLH and other states of immune dysregulation such as PID might accomplish several goals: (1) enhance primary HLH control; (2) deplete recipient T cells to improve engraftment; (3) deplete recipient antigen-presenting cells and donor T cells to decrease inflammation and GVHD. However, donor T-cell depletion might also be expected to impede engraftment and achievement of full donor chimerism. These hypotheses drove investigation of “proximal” alemtuzumab timing (days −12 to −8 before infusion) with potentially more effect on the graft vs “distal” timing (days −22 to −19) with perhaps more impact on recipient immune cells. Two single-center trials demonstrated less mixed chimerism with “distal” dosing.6,9 “Intermediate” alemtuzumab at days −14 to −10 was found to further reduce rates of mixed chimerism: subsequent cell therapy rate of 14% vs 53% after proximal and 38% after distal dosing.10
The BMT CTN study 1204 reported here is a US-based, prospective, phase 2 trial conducted at 22 sites and involving 34 children and young adults with HLH and 12 with PID (median age, 2.3 years [range, 5 months to 28 years]) who underwent a RIC fludarabine and melphalan-based HSCT with bone marrow from fully HLA-matched related or 7 to 8/8 HLA-matched unrelated donors.1 “Intermediate” dosing of alemtuzumab was used, along with cyclosporine and methylprednisolone as GVHD prophylaxis (see figure panel B). Findings were 1-year survival of 80% and 18-month survival of 67%. More than 50% of patients had graft failure or received another cellular intervention. A slightly higher than expected transplant-related mortality rate of 13% was observed, resulting from bacterial and viral infections, organ failure, and GVHD. These data provide benchmarks for further trials of HSCT in HLH and PID. Immediate next steps include optimization of alemtuzumab pharmacokinetics perhaps based on recipient lymphocyte counts and consideration of alternative GVHD prophylaxis approaches. Of note, the level of residual recipient chimerism for which additional action was taken was highly variable, and those patients who did reactivate HLH all had donor T-cell chimerism >37%. Thus, the exact relevance and priority of mixed chimerism as an end point and rational thresholds for further cell therapy after HSCT in HLH deserve further study.
Although survival was slightly lower and the incidence of mixed chimerism was higher than expected after prior single-center studies, the multicenter BMT CTN 1204 trial proves, first, how much progress has been made in survival for patients with HLH and PID and, second, how sequential single-institution and cooperative group trials can rationally address discrete questions even in very rare diseases. In addition to highlighting specific research questions related to alemtuzumab timing, immune reconstitution, and HSCT regimen design, this study lays the basis for trials of alternative haploidentical and umbilical cord donors, of novel anticytokine therapies and antibody-based conditioning, and for genetic modification of hematopoietic stem cells or T cells in hereditary diseases such as HLH and PID. The field will not rest on this “intermediate” ground!
Conflict-of-interest disclosure: The author declares no competing financial interests.