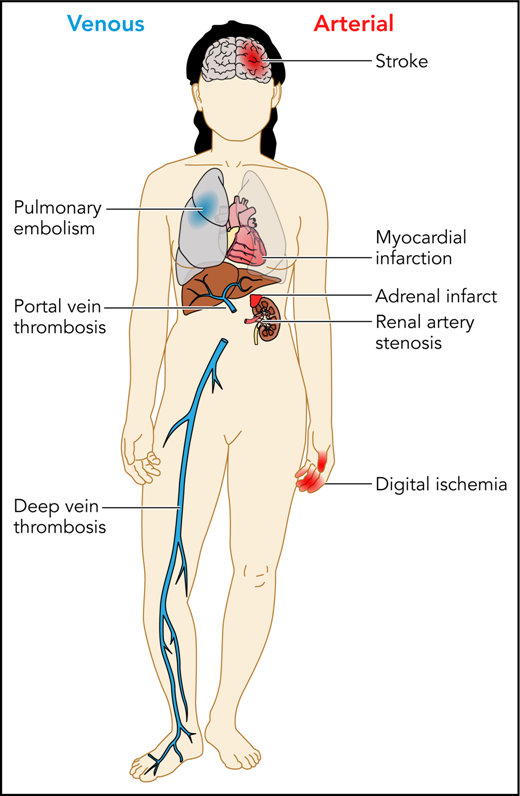
Patients with APS can experience a variety of thrombotic complications, both arterial and venous. Professional illustration by Patrick Lane, ScEYEnce Studios.
Patients with APS can experience a variety of thrombotic complications, both arterial and venous. Professional illustration by Patrick Lane, ScEYEnce Studios.
APS remains a rare and poorly understood clinical condition with potentially devastating consequences. The cardinal manifestations are arterial and venous thromboses, often occurring in younger patients, and pregnancy morbidity. Other manifestations include catastrophic APS, immune thrombocytopenia, and other more rare and potentially related conditions such as pulmonary hemorrhage (see figure).2 Although many patients with APS have coincident autoimmune conditions such as systemic lupus erythematosus, a considerable fraction present with unprovoked thromboembolism or pregnancy morbidity without other manifestations of autoimmune disease. Our understanding of this disease is further complicated by our lack of knowledge of its pathophysiology. Although various hypotheses have been proposed for the mechanism by which thrombosis and/or pregnancy loss occur, agreement on its basic biology is far from universal. The diagnosis and management of this disease is further complicated by its laboratory evaluation, which is fraught with the potential for error, both within the laboratory as a result of the vagaries of the assays used and because of the coadministration of anticoagulants which may cause false-positive or false-negative test results.3,4
Two older randomized control trials established standard-intensity warfarin as the preferred treatment for secondary prevention of arterial and venous thrombosis in patients with APS.5,6 Warfarin is difficult to use and is associated with a substantial risk of life-threatening bleeding. Thus, clinicians have sought to use direct oral anticoagulants (DOACs) in patients with APS, given the observation that they were at least as effective and as safe as warfarin in other clinical situations.7 DOACs do not need to be monitored, and they have a reduced risk of catastrophic bleeding compared with warfarin. Therefore, their use in this setting is appealing.
Pengo and colleagues are to be congratulated on their rigorously performed randomized control trial. In their study, 59 patients were randomly assigned to receive rivaroxaban and 61 to receive warfarin. All patients had had prior thrombosis (arterial and/or venous) and were triple positive, that is, they had high-titer immunoglobulin G (IgG) or IgM anticardiolipin antibodies, IgG or IgM anti-β2GP1 antibodies, and a lupus anticoagulant that demonstrated persistent positivity on 2 tests at least 12 weeks apart. This serologic profile has previously been associated with a very high risk of thromboembolism.8 The study was stopped early at the recommendation of the Adjudication and Safety Committee because it was observed that the composite primary outcome (thromboembolic events, major bleeding, and vascular death) occurred in 11 patients allocated to rivaroxaban and 2 patients allocated to warfarin (hazard ratio, 6.7; 95% confidence interval, 1.5-30.5; P = .01). A post hoc intention-to-treat analysis identified 2 additional outcome events in the rivaroxaban group, further worsening the imbalance. Of the 11 events identified in the on-treatment analysis, 4 were ischemic stroke, 3 were myocardial infarctions, and 4 were major bleeds in the patients allocated to rivaroxaban, whereas in the warfarin arm, there were 2 patients with major bleeding and no recurrent thrombotic events.
Studies in patients with APS are difficult to perform because the condition is rare, and patients suffer from a spectrum of clinical disorders, only some of which will qualify them for studies of anticoagulants (eg, patients may present with both new thromboses and profound thrombocytopenia or may have exclusively pregnancy morbidity). Furthermore, patients tend to be treated by a variety of clinicians depending on their initial presentation, which makes accruing patients to a study problematic. Finally, there are strongly held opinions regarding the most appropriate treatment of these patients, which further limits our ability to perform rigorous studies. However, such studies have been performed, most notably, a randomized control trial in which patients were treated with the same dose of rivaroxaban and had a much lower rate of identified complications.9
How can we reconcile the studies that are available? Patients suspected of having APS should have the diagnosis confirmed by objective testing performed in expert laboratories. A large proportion of patients, perhaps a majority of them labeled as having APS, do not in fact have the disorder, either because the testing was inadequate (usually only a single test that demonstrated a low-titer anticardiolipin antibody without confirmation) or because they were tested while they were receiving anticoagulants. These patients who do not in fact have the disorder represent a low-risk group for recurrent thrombosis and are probably adequately treated with a DOAC at the usual therapeutic dose or with warfarin with a target international normalized ratio (INR) of 2.0 to 3.0. Patients with true APS with a more profound prothrombotic state are probably at particularly high risk of thrombosis. The evidence provided by Pengo and colleagues suggests that these patients are best treated with warfarin administered to achieve a target INR of 2.0 to 3.0. Whether aspirin should be added to the treatment for those patients who had prior arterial thrombosis remains unknown. Evidence from the trial reported by Pengo et al supports other less rigorous analyses that suggest that aspirin added to warfarin may be beneficial in some patients.
What research is now required? Dose intensification of DOACs might be considered, and the use of DOACs combined with an antiplatelet agent might also be considered. However, such strategies would require testing in adequately powered clinical trials. Until such studies are performed, this study reaffirms that warfarin administered with a target INR of 2.0 to 3.0 remains the standard for the prevention of thrombosis in higher-risk patients with APS.
Conflict-of-interest disclosure: M.C. has sat on data safety monitoring boards for Daiichi and Bayer; has received personal funding for advisory boards for Shionogi, Octapharma, BMS Canada, and Servier Canada; has prepared educational material and/or presented on behalf of Alexion, Pfizer, and CSL Behring; and his institution has received research funding on his behalf from Bayer, Leo Pharma, and the Heart and Stroke Foundation. The remaining author declares no competing financial interests.