TO THE EDITOR:
Pericardial effusion in patients with Hodgkin lymphoma (HL) occurs in 5% to 24% of patients at diagnosis.1-3 However, little is known about the incidence, clinical characteristics, and outcomes for these patients. Most cases are clinically silent, and the effusion resolves with treatment of the underlying malignancy. In rare cases, the pericardial effusion leads to pericardial tamponade and requires immediate intervention.4 Pericardial effusion in HL arises because of blockage of venous and lymphatic circulation of pericardial fluid secondary to lymphatic or hematogenous metastasis to the pericardium.5
The clinical outcome of patients with pericardial effusion at diagnosis in HL has never been studied in a large trial. Children’s Oncology Group (COG) protocol AHOD0031 offered an opportunity to evaluate pericardial effusion as an adverse risk factor for a large cohort of intermediate-risk patients treated with a standard backbone of chemotherapy.6 COG AHOD0031 was a randomized, response-based, centrally reviewed protocol, which enrolled 1711 eligible children with intermediate-risk HL from September 2002 to July 2009. Its primary aim was to test the paradigm of response-based therapy to maintain excellent cure rates while avoiding treatment-associated risks that compromise long-term health.6 Patients who were eligible included those younger than age 22 years with newly diagnosed, biopsy-proven HL, Ann Arbor7 stages IB, IAE, IIB, IIAE, IIIA, and IVA with or without bulk disease and IA or IIA with bulk disease. This trial was registered at https://clinicaltrials.gov/ as NCT00025259 and was approved by the National Cancer Institute and participating institutional review boards. Here we retrospectively review the incidence, clinical characteristics, and outcome of pediatric patients with intermediate-risk HL and pericardial effusion at diagnosis treated on COG AHOD0031.
Patients received 2 cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) followed by response evaluation of rapid early responder (RER) vs slow early responder (SER) status. SERs were randomly assigned to an additional 2 cycles of ABVE-PC with or without 2 cycles of dexamethasone, etoposide, cisplatin, and cytarabine (DECA), and all received 21 Gy involved field radiotherapy (IFRT). RERs received 2 additional cycles of ABVE-PC. RERs with complete response (CR) were randomly assigned to IFRT or no further therapy. RERs without CR were non-randomly assigned to IFRT.
Of the 1711 patients enrolled on the protocol, 1423 had adequate available imaging to quantitate pericardial effusion. Only imaging studies that had been downloaded to the computer system were reviewed; studies on hard copy films or discs were excluded. There was no difference in baseline characteristics or outcomes between patients with or without available imaging. Imaging, including a contrast computed tomography (CT) scan of the neck, chest, abdomen, and pelvis was obtained before chemotherapy was initiated. In this retrospective analysis, pericardial effusions were reviewed by 1 radiologist blinded to results. The pericardial effusions were categorized as small (posterior location along the left ventricle and left atrium), moderate (anterior location along the right ventricle), or large (surrounding the heart).8 Moderate and large pericardial effusions were recorded and correlated with disease stage, bulk, histology, tumor response, presence of pleural effusion, response to IFRT, and survival. Events included relapse or progression, subsequent malignant neoplasm (SMN), and death.
Overall, 288 (20.2%) of the 1423 patients with adequate imaging had pericardial effusion at diagnosis; 87 (6.1%) of the effusions were moderate or large, and 201 (14.1%) were trace or small. Only those patients with moderate or large pericardial effusions were evaluated as having an effusion, which helped keep the focus of the study on the group with potential clinical relevance. Our reported incidence of pericardial effusion is similar to that previously reported in adults3 but is greater than that reported in another pediatric study (5%) of fewer patients.1 A demographic comparison of patients with moderate or large pericardial effusion (n = 87) and those without (n = 1336) is provided in Table 1. Compared with patients without pericardial effusion, patients with pericardial effusion were older (P = .0075), more often female (P = .023), and more likely to have B symptoms (P = .0009), bulk (P = .027), large mediastinal adenopathy (P < .0001), or nodular sclerosing histology (P = .0076), with no difference in stage.
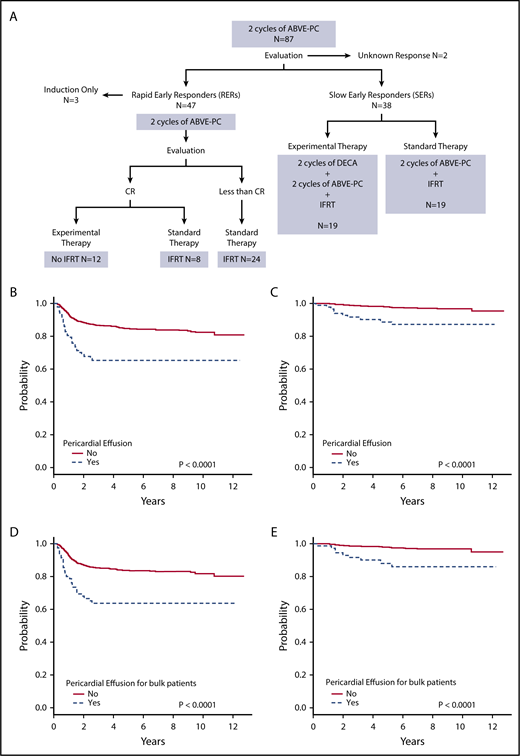
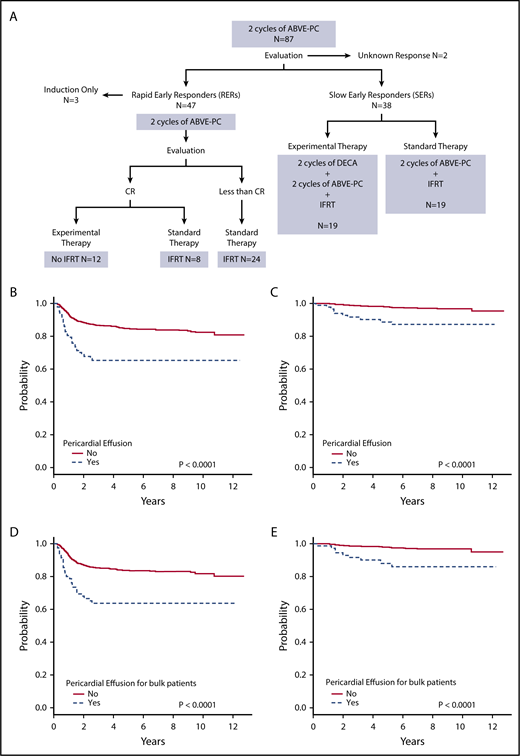
Figure 1A shows the treatment assignment of all patients with pericardial effusion treated on study AHOD0031. Event-free survival (EFS) and overall survival (OS) were worse for patients with pericardial effusion compared with those without (both P < .0001) (Figure 1B-C). The 5-year EFS for pericardial effusion–positive and –negative groups was 65.2% (95% confidence interval [CI], 53.7%-76.7%) and 84.6% (95% CI, 82.3%-87.0%), respectively. Corresponding OS was 88.7% (95% CI, 80.8%-96.5%) and 98.0% (95% CI, 97.0%-98.9%). For patients with pericardial effusion, stage did not have an impact on tumor response or survival. EFS and OS were worse for patients with bulk disease and pericardial effusion compared with those with bulk disease alone (both P < .0001) (Figure 1D-E). For patients with bulk disease, 5-year EFS was 63.6% (95% CI, 50.9%-76.3%) for those with pericardial effusion and 83.7% (95% CI, 81.0%-86.5%) for those without. Corresponding OS was 88.0% (95% CI, 79.2%-96.8%) and 98.1% (95% CI, 97.0%-99.1%). Thirty patients (34.5%) with pericardial effusion also had pleural effusion. There was no significant difference between those with and without pleural effusion for patients with pericardial effusion in terms of EFS (P = .15) and OS (P = .31).
Treatment and outcomes of patients with pericardial effusion at diagnosis. (A) Treatment of patients with pericardial effusion on AHOD0031. (B) EFS and (C) OS for patients with and without pericardial effusion. (D) EFS and (E) OS for patients with bulk disease with and without pericardial effusion.
Treatment and outcomes of patients with pericardial effusion at diagnosis. (A) Treatment of patients with pericardial effusion on AHOD0031. (B) EFS and (C) OS for patients with and without pericardial effusion. (D) EFS and (E) OS for patients with bulk disease with and without pericardial effusion.
Thirty-eight patients (43.7%) with pericardial effusion were SERs, whereas only 226 (16.9%) of 1336 patients without effusion were SERs (P < .0001). Overall, AHOD0031 demonstrated better EFS and OS for RERs compared with SERs.6 However, for patients with pericardial effusion, there was no difference in EFS (P = .58) or OS (P = .71) for RERs compared with SERs. As was seen for all patients who participated in AHOD0031, patients with pericardial effusion who were RERs with CR did not show a statistically significant benefit from IFRT.
There were 30 first events among patients with pericardial effusion, including 28 relapses, 1 SMN, and 1 death. All events occurred within 3 years after the patient ended therapy.
To evaluate whether pericardial effusion is a primary prognostic factor or secondary to other factors, we explored whether patients with pericardial effusion would have been identified as high-risk on the basis of additional clinical risk factors as defined by their Childhood Hodgkin International Prognostic Score (CHIPS).9 The CHIPS consists of 4 elements worth 1 point each: stage 4 disease, large mediastinal adenopathy, albumin <3.5 g/dL, and fever. CHIPS ≥2 is considered high-risk. Because AHOD0031 was a study of patients with intermediate risk, the highest possible CHIPS was 3. Of the 87 patients with pericardial effusions, 9 had CHIPS of 1, 58 had CHIPS of 2, and 20 had CHIPS of 3. Patients with CHIPS of 1 had better outcomes than patients with CHIPS of 2 or 3, although there was no statistically significant difference in EFS (P = .16) and OS (P = .25). A Cox multivariable analysis was performed that considered the following criteria: moderate or large pericardial effusion, age at diagnosis, weight loss, sex, histology, response stratum, and individual CHIPS elements. Pericardial effusion (hazard ratio [HR], 2.16; 95% CI, 1.43-3.25; P = .0002), age (HR, 1.06; 95% CI, 1.01-1.11; P = .02), fever (HR, 1.98; 95% CI, 1.31-3.00; P = .001), albumin (HR, 0.78; 95% CI, 0.63-0.96; P = .02), and RER status (HR, 0.70; 95% CI, 0.51-0.96; P = .03) were found to be statistically significant prognostic factors.
Although pericardial effusion is often clinically silent and improves with chemotherapy, it is an independent indicator of poor prognosis in children and adolescents with intermediate-risk HL. The multivariable Cox regression analysis indicates that the presence of a pericardial effusion plays a significant role in EFS when other risk factors are considered. Small numbers of study participants preclude a definitive conclusion, but this represents an area of future research to determine whether patients with moderate or large pericardial effusion at diagnosis may benefit from more intensive therapy.
Acknowledgments
The authors thank the staff at the Quality Assurance Review Center for their support on this project.
This work was supported by grants from the National Cancer Institute, National Institutes of Health to the COG Chair (U10CA98543) and the COG Statistics and Data Center (U10CA098413), the National Clinical Trials Network (NCTN) Operations Center (U10CA180886), the NCTN Statistics and Data Center (U10CA180899), and the St. Baldrick’s Foundation.
Authorship
Contribution: L.J.M., K.M.M., and K.M.K. conceived of and designed the study; D.L.F., C.L.S., and K.M.K. provided study materials or patients; L.J.M., K.M.M., Q.P., and K.M.K. collected and assembled data; and all authors participated in the analysis and interpretation of data and the drafting and critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lianna J. Marks, Department of Pediatrics, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 139, New York, NY 10065; e-mail: lianna.marks@gmail.com.