Key Points
The fusion between FVIII and anti-VWF nanobodies increases affinity for VWF 25-fold without compromising FVIII activity.
Stabilized VWF binding results in a twofold enhanced circulatory survival of FVIII and reduced anti-FVIII antibody formation.
Abstract
Von Willebrand factor (VWF) modulates factor VIII (FVIII) clearance and the anti-FVIII immune response. Despite the high affinity that defines the FVIII/VWF interaction, association/dissociation kinetics dictates 2% to 5% FVIII being present as free protein. To avoid free FVIII when studying the FVIII-VWF complex in vivo, we designed a FVIII-nanobody fusion protein, with the nanobody part being directed against VWF. This fusion protein, designated FVIII-KB013bv, had a 25-fold higher affinity compared with B-domainless FVIII (BDD-FVIII) for VWF. In vitro analysis revealed full cofactor activity in 1-stage clotting and chromogenic assays (activity/antigen ratio 1.0 ± 0.3 and 1.1 ± 0.3, respectively). In vivo, FVIII-013bv displayed a twofold increased mean residence time compared with BDD-FVIII (3.0 hours vs 1.6 hours). In a tail clip–bleeding assay performed 24 hours after FVIII infusion, blood loss was significantly reduced in mice receiving FVIII-KB013bv vs BDD-FVIII (15 ± 7 μL vs 194 ± 146 μL; P = .0043). Unexpectedly, when examining anti-FVIII antibody formation in FVIII-deficient mice, the immune-response toward FVIII-KB013bv was significantly reduced compared with BDD-FVIII (1/8 vs 14/16 mice produced anti-FVIII antibodies after treatment with FVIII-KB013bv and BDD-FVIII, respectively). Our data show that a stabilized interaction between FVIII and VWF is associated with a prolonged survival of FVIII and a reduced immune response against FVIII.
Introduction
Factor VIII (FVIII) is a protein pre-cofactor critical to coagulation. Upon secretion, FVIII circulates bound to von Willebrand factor (VWF), which is important to stabilize FVIII and to prevent its premature degradation and clearance.1-5 VWF also modulates anti-FVIII antibody development in hemophilia A patients, in part by regulating FVIII uptake by antigen-presenting cells.6-8 Noteworthy, FVIII is endocytosed following binding of the VWF-FVIII complex to the surface of antigen-presenting cells, whereas VWF is protected from internalization.9
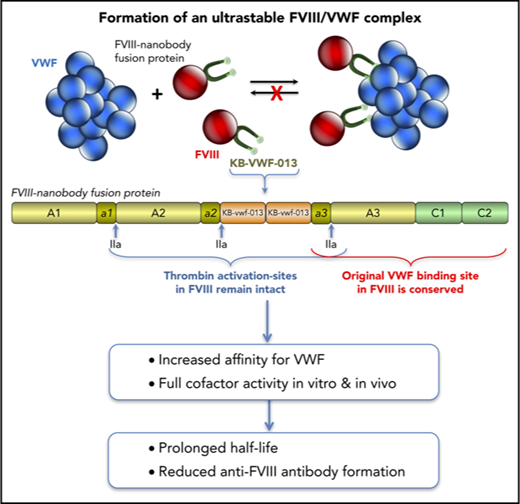
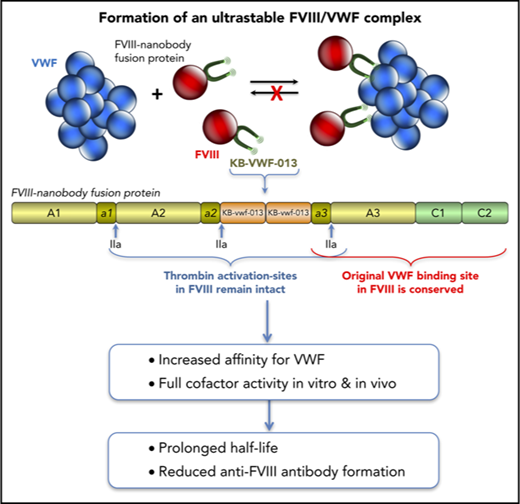
The molecular basis of VWF-FVIII complex formation is elucidated in great detail, involving interactions between the VWF D′-D3 region and 2 regions within FVIII: the acidic a3-region including sulfated-Tyr1680 (Tyr1699 in HGVS-numbering) and the C-terminal C1-C2 domains.10-12 Two percent to 5% of FVIII circulates as free protein despite its high affinity for VWF (0.2-1.5 nM) and is cleared faster than VWF-bound FVIII.12-18 The free FVIII pool is maintained via continuous release of FVIII from the FVIII-VWF complex.19 Investigating the VWF-FVIII complex in vivo is therefore complicated by the constitutive presence of free FVIII, which displays differential clearance and uptake by antigen-presenting cells compared with VWF-bound FVIII. To minimize the contribution of free FVIII, we designed a FVIII-variant containing a nanobody against the VWF D′-D3 domain. Promoted by this nanobody, an ultrastable FVIII-VWF complex is formed, resulting in a prolonged FVIII survival and reduced anti-FVIII immune response.
Study design
An extensive description of the experimental procedures is given in the supplemental materials, available on the Blood Web site.
Clearance
F8-deficient mice were given FVIII (250 U/kg), and residual FVIII activity was determined using a chromogenic assay in plasma taken between 3 minutes and 48 hours.
Tail clip assay
F8-deficient mice were given FVIII (500 U/kg), and after 24 hours, the terminal 3 mm of the tail was amputated. Blood loss was monitored over 30 minutes.
Formation of anti-FVIII antibodies
F8-deficient mice were given 4 FVIII injections (50 U/kg) at weekly intervals, and a fifth injection at day 40. The presence of anti-FVIII antibodies was analyzed in plasma taken at day 43.
Results and discussion
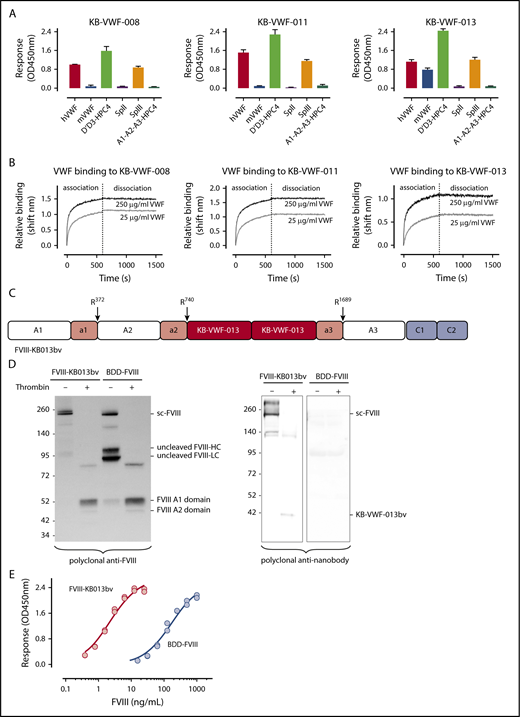
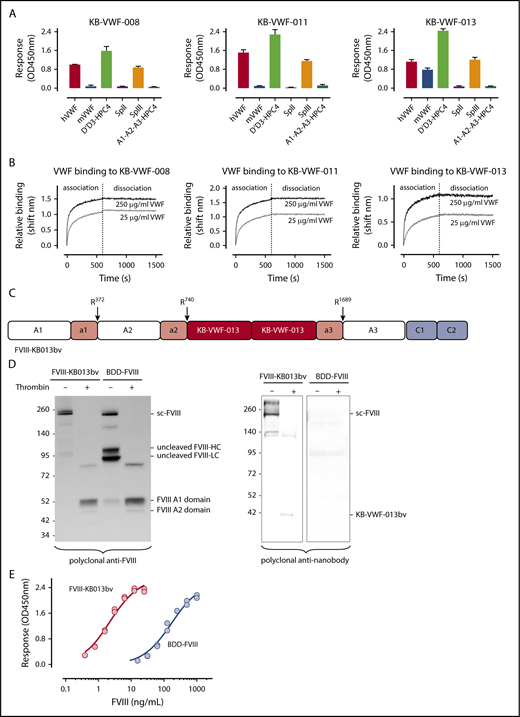
To improve FVIII-VWF complex formation, we used the notion that antibodies often dissociate slowly, and that small-sized nanobodies display high-affinity antigen binding. From our library of anti-VWF nanobodies,20 we isolated 3 nanobodies that recognize the FVIII-binding D′D3 region (Figure 1A), while leaving FVIII binding to VWF unaffected. These nanobodies have slow dissociation rates (apparent dissociation rate constants [koff,app]: 2.0 ± 1.1 × 10−5 s−1, 0.6 ± 0.5 × 10−5 s−1, and 2.2 ± 1.2 × 10−5 s−1 for KB-VWF-008, -011, and -013, respectively; Figure 1B). These values are ∼100-fold lower compared with the average koff, reported in different studies for the FVIII-VWF interaction (ie, koff,app = 2.2 ± 0.3 × 10−3 s−1, range 2.3 × 10−4 s−1 to 6.6 × 10−3 s−1).16-18,21,22
Anti-VWF nanobodies in a FVIII-nanobody fusion protein. (A) Immobilized nanobodies (5 μg/mL) were incubated with humanVWF, murineVWF, proteolytic fragment SpII (ie, VWF residues 2129-2813), fragment SpIII (residues 764-2128), D′-D3-HPC4 (residues 764-1247) and A1-A2-A3-HPC4 (residues 1260-1874). Bound proteins were probed using polyclonal anti-VWF antibodies or with monoclonal antibody HPC4 and detected via peroxidase-mediated hydrolysis of 3-3′-5-5′-tetramethylbenzidine. Plotted are optical density values corrected for binding of the proteins to albumin-coated wells (mean ± SD; n = 3). (B) Association and dissociation of humanVWF (25 μg/mL, gray lines; or 250 μg/mL, black lines) to immobilized nanobodies (10 μg/mL) was analyzed via BLI-analysis using Octet-QK equipment. Association was allowed for 600 seconds, and dissociation was monitored for 900 seconds. Representative sensorgrams for each nanobody are presented. (C) Schematic representation of FVIII-KB013bv, in which FVIII B-domain residues Gln744-Arg1648 are replaced by 2 copies of nanobody KB-VWF-013. Original thrombin activation sites and VWF binding sites are conserved in this protein, whereas Arg1648 is no longer present. (D) Purified FVIII-KB013bv and BDD-FVIII (1 μg/mL) were incubated in the absence or presence of thrombin (10 nM) for 30 minutes at 37°C and analyzed via western blotting using polyclonal anti-FVIII and anti-nanobody antibodies. Sc-FVIII, single chain FVIII; FVIII-HC, FVIII heavy chain; FVIII-LC, FVIII light chain. (E) Wells coated with purified VWF (10 μg/mL) were incubated with FVIII-KB013bv (0-25 ng/mL; red symbols) or BDD-FVIII (0-1 μg/mL; blue symbols). Bound FVIII was probed using peroxidase-labeled monoclonal anti-FVIII antibody 833 and detected via peroxidase-mediated hydrolysis of 3-3′-5-5′-tetramethylbenzidine. Plotted are typical binding curves of an experiment performed in duplicate. Half-maximal binding values were calculated from 3 independent experiments and were 4.0 ± 2.1 ng/mL and 170 ± 22 ng/mL for FVIII-KB013bv and BDD-FVIII, respectively.
Anti-VWF nanobodies in a FVIII-nanobody fusion protein. (A) Immobilized nanobodies (5 μg/mL) were incubated with humanVWF, murineVWF, proteolytic fragment SpII (ie, VWF residues 2129-2813), fragment SpIII (residues 764-2128), D′-D3-HPC4 (residues 764-1247) and A1-A2-A3-HPC4 (residues 1260-1874). Bound proteins were probed using polyclonal anti-VWF antibodies or with monoclonal antibody HPC4 and detected via peroxidase-mediated hydrolysis of 3-3′-5-5′-tetramethylbenzidine. Plotted are optical density values corrected for binding of the proteins to albumin-coated wells (mean ± SD; n = 3). (B) Association and dissociation of humanVWF (25 μg/mL, gray lines; or 250 μg/mL, black lines) to immobilized nanobodies (10 μg/mL) was analyzed via BLI-analysis using Octet-QK equipment. Association was allowed for 600 seconds, and dissociation was monitored for 900 seconds. Representative sensorgrams for each nanobody are presented. (C) Schematic representation of FVIII-KB013bv, in which FVIII B-domain residues Gln744-Arg1648 are replaced by 2 copies of nanobody KB-VWF-013. Original thrombin activation sites and VWF binding sites are conserved in this protein, whereas Arg1648 is no longer present. (D) Purified FVIII-KB013bv and BDD-FVIII (1 μg/mL) were incubated in the absence or presence of thrombin (10 nM) for 30 minutes at 37°C and analyzed via western blotting using polyclonal anti-FVIII and anti-nanobody antibodies. Sc-FVIII, single chain FVIII; FVIII-HC, FVIII heavy chain; FVIII-LC, FVIII light chain. (E) Wells coated with purified VWF (10 μg/mL) were incubated with FVIII-KB013bv (0-25 ng/mL; red symbols) or BDD-FVIII (0-1 μg/mL; blue symbols). Bound FVIII was probed using peroxidase-labeled monoclonal anti-FVIII antibody 833 and detected via peroxidase-mediated hydrolysis of 3-3′-5-5′-tetramethylbenzidine. Plotted are typical binding curves of an experiment performed in duplicate. Half-maximal binding values were calculated from 3 independent experiments and were 4.0 ± 2.1 ng/mL and 170 ± 22 ng/mL for FVIII-KB013bv and BDD-FVIII, respectively.
Although KB-VWF-011 had the lowest koff,app, KB-VWF-013 was fused with FVIII, because its cross-reactivity with murine VWF facilitates in vivo experiments. In this fusion protein, named FVIII-KB013bv, 2 copies of KB-VWF-013 are replacing B-domain residues Gln744-Arg1648, allowing FVIII to be secreted as a single chain protein (Figure 1C-D; supplemental Figure 1). Natural thrombin-activation sites at Arg372, Arg740, and Arg1689 are maintained. Consequently, thrombin generates similar proteolytic products upon activation of FVIII-KB013bv as for B-domainless FVIII (BDD-FVIII), whereas the nanobody portion is released (Figure 1D). Probably, this nanobody portion remains bound to VWF in vivo. FVIII-KB013bv exhibits full cofactor activity in 1-stage clotting and chromogenic activity assays (activity/antigen ratios: 1.0 ± 0.3 [n = 5] and 1.1 ± 0.3 [n = 5], respectively; supplemental Table 1). When comparing VWF binding in an enzyme-linked immunosorbent assay, FVIII-KB013bv was ∼40-fold more efficient than BDD-FVIII, with half-maximal binding being 4.0 ± 2.1 ng/mL (2.1 ± 1.1 × 10−11 nM) and 170 ± 22 ng/mL (1.1 ± 0.1 × 10−9 nM; Figure 1E), respectively. Kinetic measurements revealed an apparent affinity constant of 13 ± 11 pM, 25-fold more efficient compared with BDD-FVIII (330 ± 127 pM; supplemental Figure 2). Using this affinity, we calculated that 0.14% of FVIII-KB013bv is present as free protein in plasma, compared with 3.3% of FVIII (supplemental materials). Binding of FVIII-KB013bv but not BDD-FVIII to VWF was preserved after introduction of a Tyr1680Phe mutation (supplemental Figure 3), which eliminates natural VWF binding in FVIII. Thus, FVIII-KB013bv has full cofactor activity in vitro and binds more efficiently than BDD-FVIII to VWF.
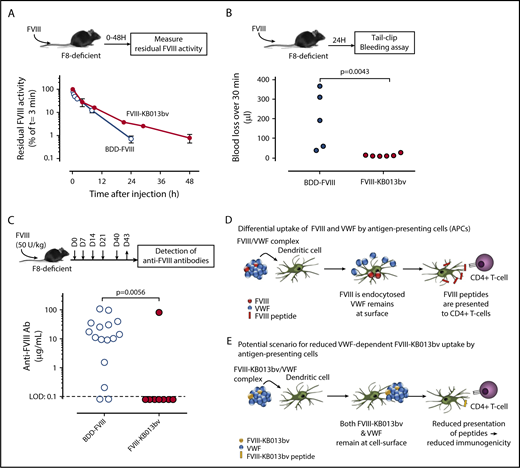
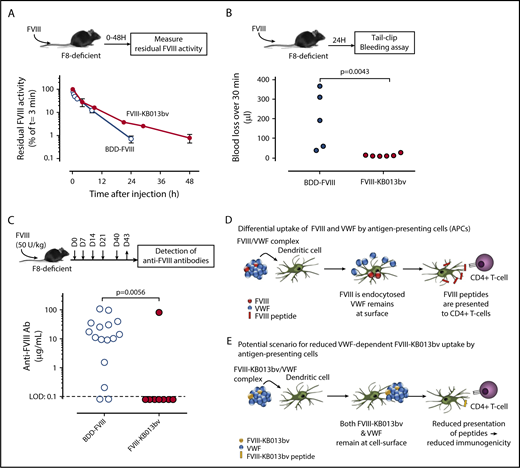
We then tested survival of FVIII-KB013bv in F8-deficient mice that have normal VWF:Ag levels (supplemental Figure 4). Compared with BDD-FVIII, the mean residence time for FVIII-KB013bv was increased about twofold (1.6 hours [95% confidence interval: 1.3-2.1 hours] and 3.0 hours [95% confidence interval 2.4-4.2 hours] for BDD-FVIII and FVIII-KB013bv, respectively; P = .044; Figure 2A). Because FVIII-KB013bv remains associated with VWF, these data support the view that FVIII is cleared as part of the FVIII-VWF complex rather than as free protein.1,23 The prolonged survival was confirmed in a tail clip assay performed 24 hours after injection. In our hands, this model requires 0.2 U/mL FVIII to arrest bleeding, and according to the pharmacokinetic parameters, BDD-FVIII levels are ∼0.07 U/mL compared with 0.27 U/mL for FVIII-KB013bv 24 hours after infusion of 500 U/kg. Indeed, blood loss was significantly higher in BDD-FVIII vs FVIII-KB013bv treated mice (194 ± 146 μL [n = 5] vs 15 ± 7 μL [n = 6]; mean ± standard deviation [SD]; P = .0043; Figure 2B). Importantly, prolonged expression of FVIII-KB013bv at supraphysiological levels (4 U/mL) did not induce an increase in thrombotic makers (D-dimer, thrombin-antithrombin complexes; supplemental Figure 5). Apparently, improved FVIII-VWF binding is associated with prolonged circulatory survival of FVIII, and FVIII-KB013bv is functionally active in vivo. Furthermore, the nanobody fragment that remains bound to VWF does not seem to impair VWF function.
In vivo analysis of FVIII-KB013bv. (A) FVIII-deficient mice were given BDD-FVIII (open circles) or FVIII-KB013bv (closed circles) at a dose of 250 U/kg via tail vein injection; then, blood samples were taken at various time points, and plasma was analyzed for residual FVIII activity. Plotted are FVIII activities relative to the activity at t = 3 minutes, which was arbitrarily set at 100%. Each data point represents mean ± SD of 3 to 5 mice, and each mouse was bled 1 to 2 times. (B) FVIII-deficient mice received BDD-FVIII or FVIII-KB013bv (500 U/kg) via tail vein injection, and 24 hours after injection a tail clip–bleeding assay was performed. Clipped tails were immersed in saline at 37°C, and blood was collected for 30 minutes. Blood loss for each individual mouse is indicated. (C) Mice were given BDD-FVIII or FVIII-KB013bv (50 U/kg) at days 0, 7, 14, 21, and 40 via tail vein injection. At day 43, blood samples were taken, and plasma was analyzed for the presence of murine anti-FVIII antibodies. Briefly, wells coated with BDD-FVIII were incubated with murine plasma, and bound murine antibodies were detected via peroxidase-labeled polyclonal goat anti-mouse antibodies. As standard, a monoclonal anti-FVIII antibody was used. The limit of detection (LOD) in this assay was 0.1 μg/mL. The immune response for each individual mouse is presented. Statistical analyses were performed using a Mann-Whitney U test. (D) According to Sorvillo et al,9 the VWF-FVIII complex separates at the cellular surface of antigen-presenting cells, with FVIII being endocytosed and most of the VWF molecules remaining outside the cell. (E) Based on the model described by Sorvillo et al, we anticipate that the complex between FVIII-KB013bv and VWF will not dissociate, and consequently, there will be reduced uptake of FVIII-KB013bv by antigen-presenting cells. Fewer FVIII-derived peptides will then be presented to T cells, and in turn, there will be reduced development of anti-FVIII antibodies.
In vivo analysis of FVIII-KB013bv. (A) FVIII-deficient mice were given BDD-FVIII (open circles) or FVIII-KB013bv (closed circles) at a dose of 250 U/kg via tail vein injection; then, blood samples were taken at various time points, and plasma was analyzed for residual FVIII activity. Plotted are FVIII activities relative to the activity at t = 3 minutes, which was arbitrarily set at 100%. Each data point represents mean ± SD of 3 to 5 mice, and each mouse was bled 1 to 2 times. (B) FVIII-deficient mice received BDD-FVIII or FVIII-KB013bv (500 U/kg) via tail vein injection, and 24 hours after injection a tail clip–bleeding assay was performed. Clipped tails were immersed in saline at 37°C, and blood was collected for 30 minutes. Blood loss for each individual mouse is indicated. (C) Mice were given BDD-FVIII or FVIII-KB013bv (50 U/kg) at days 0, 7, 14, 21, and 40 via tail vein injection. At day 43, blood samples were taken, and plasma was analyzed for the presence of murine anti-FVIII antibodies. Briefly, wells coated with BDD-FVIII were incubated with murine plasma, and bound murine antibodies were detected via peroxidase-labeled polyclonal goat anti-mouse antibodies. As standard, a monoclonal anti-FVIII antibody was used. The limit of detection (LOD) in this assay was 0.1 μg/mL. The immune response for each individual mouse is presented. Statistical analyses were performed using a Mann-Whitney U test. (D) According to Sorvillo et al,9 the VWF-FVIII complex separates at the cellular surface of antigen-presenting cells, with FVIII being endocytosed and most of the VWF molecules remaining outside the cell. (E) Based on the model described by Sorvillo et al, we anticipate that the complex between FVIII-KB013bv and VWF will not dissociate, and consequently, there will be reduced uptake of FVIII-KB013bv by antigen-presenting cells. Fewer FVIII-derived peptides will then be presented to T cells, and in turn, there will be reduced development of anti-FVIII antibodies.
VWF may reduce the development of anti-FVIII antibodies.6,7 However, both free and VWF-bound FVIII are present in in vivo studies, making it difficult to distinguish whether free or VWF-bound FVIII favors antibody development. We tested if improved complex formation affects inhibitor development. FVIII-deficient mice received repeated doses of BDD-FVIII or FVIII-KB013bv (50 U/kg; Figure 2C). Of note, dosing was based on units activity/weight basis. Because BDD-FVIII and FVIII-KB013bv have different molecular weights (166 kDa vs 196 kDa), this may have resulted in different protein dosing per mouse. The presence of anti-FVIII antibodies was detected in 14 of 16 mice (87.5%) treated with BDD-FVIII, with a median titer of 12.7 μg/mL (Figure 2C). In contrast, only 1 mouse out of 8 (12.5%) treated with FVIII-KB013bv developed anti-FVIII antibodies (P = .0056 vs BDD-FVIII). A Bethesda assay was used to measure inhibitory antibodies in a subset of the samples (supplemental Figure 6). Mice having inhibitory antibodies were also positive in the enzyme-linked immunosorbent assay. The presence of anti-nanobody antibodies was not detected in any of the mice (treated with BDD-FVIII or FVIII-KB013bv). These data suggest that enhanced VWF binding may result in reduced anti-FVIII antibody formation.
When the FVIII-VWF complex binds to antigen-presenting cells, VWF predominantly remains at the cell surface, whereas FVIII is internalized and presented by the major histocompatibility complex II (Figure 2D).7,9 Our data suggest that improved VWF binding prevents internalization and subsequent presentation of FVIII by the major histocompatibility complex II (Figure 2E). These data obviously need further validation in higher animals, given that receptor expression patterns may differ between species. Our data are further compatible with the immunogenic pathway (involving, eg, CD206, but not LRP1), being different from the catabolic pathway (involving LRP1 among others, but not CD206).5,24,25 Why receptors contribute to FVIII uptake in a cell-type specific manner is unknown, but deserves further studies.
Together, our data indicate that increased VWF-binding favors FVIII survival and reduces anti-FVIII antibody formation. From a therapeutic perspective, it would be interesting to combine this FVIII-nanobody fusion protein with long-acting VWF (eg, PEGylated-VWF) to further prolong FVIII half-life, perhaps beyond the 1.5- to 1.8-fold limitation that is observed with current FVIII variants with an extended half-life (FVIII-Fc and PEG-FVIII).1
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Amine Bazaa and Amelie Harel for their contribution in the early stage of the study.
This study was supported by a Proof-of-Concept grant from INSERM-Transfert (Paris, France).
Authorship
Contribution: V.M., F.M., G.A., C.K., and P.L. performed experiments and analyzed data; C.C. and V.P. provided important intellectual input; O.D.C., C.V.D., and P.J.L. designed and supervised the study and analyzed data; and P.J.L. wrote the manuscript; and all the authors contributed to its editing.
Conflict-of-interest disclosure: G.A., O.D.C., C.V.D., and P.J.L. are inventors on a patent application involving FVIII-KB013bv. The remaining authors declare no competing financial interests.
Correspondence: Cécile V. Denis, INSERM U1176, 80 rue du General Leclerc, 94276 Le Kremlin-Bicêtre, France; e-mail: cecile.denis@inserm.fr.