In this issue of Blood, Miesbach et al show that adeno-associated virus-5 (AAV5) liver-directed gene therapy in severe and moderate hemophilia B was clinically effective, with patients achieving stable factor IX (FIX) expression. The procedure was well tolerated and no immune responses were detected.1
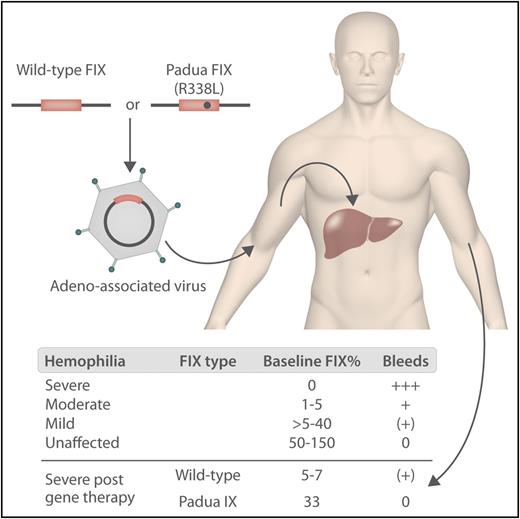
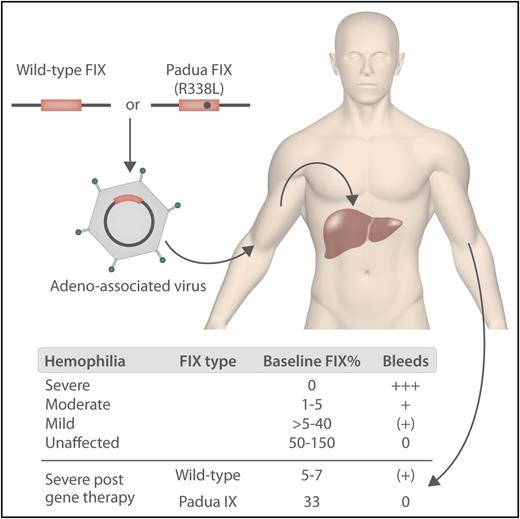
In hemophilia B gene therapy, either wild-type or Padua factor IX with a liver-specific promoter is inserted into the genome of the AAV vector and administered via a peripheral vein. The vector transduces hepatocytes, leading to the production of FIX. The levels of FIX in patients and unaffected individuals before and after gene therapy are shown below. Professional illustration by Somersault18:24.
In hemophilia B gene therapy, either wild-type or Padua factor IX with a liver-specific promoter is inserted into the genome of the AAV vector and administered via a peripheral vein. The vector transduces hepatocytes, leading to the production of FIX. The levels of FIX in patients and unaffected individuals before and after gene therapy are shown below. Professional illustration by Somersault18:24.
The introduction of clotting factor concentrates in the 1970s, and the ability to infuse them at home, revolutionized the lives of those with hemophilia. The past 30 years have witnessed the development of recombinant products devoid of human or animal proteins and with half-lives significantly longer than those of the natural proteins. The current gold standard treatment of severe disease is prophylaxis with infusions twice a week for hemophilia A and once every 1 to 2 weeks for hemophilia B. It is now possible for people with hemophilia to lead normal lives with virtually no bleeds.
In parallel with the rapid progress in IV infused treatments, a revolution has been taking place with novel products and gene therapy that is now reaching maturity. Most individuals with hemophilia have been looking forward to the time when they could be free of the burden of regular IV infusions; this is now within reach.
The breakthrough in modern era gene therapy for hemophilia was the publication of the Nathwani et al study in 2011.2 They used wild-type FIX in AAV8 vector containing a liver-specific promoter enabling peripheral administration. Although the levels of FIX achieved with the highest vector dose of 2 × 1012 vector genomes/kg were modest (mean, 5.1%), they have been maintained with no safety issues and some patients have been able to discontinue prophylaxis.2,3 Four of the 6 patients in the highest dose cohort experienced a rise in alanine aminotransferase with a drop in FIX expression in the presence of capsid-related T-cell reactivity and responded to short courses of steroids.2,3
At the end of 2017, 2 further gene therapy studies were published reporting achievement of FVIII/IX levels beyond the expectations of most treaters.4,5 George et al also used an AAV vector containing the FIX gene with the Padua mutation and achieved FIX levels of ∼33% (see figure).4 The Padua FIX mutation was previously shown to be associated with five- to 10-fold higher expression of FIX in vitro.6 Even more impressive are the results in hemophilia A where, using an AAV5 vector with a B-domain deleted FVIII, 6 of the 7 patients with hemophilia in the highest dose cohort studied achieved normal FVIII levels.5
In this issue of the journal, Miesbach et al report their experience with an AAV5 vector in hemophilia B patients with baseline FIX ≤2.0 IU/dL (≤2%) achieving mean FIX levels of 4.4% in the group receiving 5 × 1012 vector genomes/kg and 6.9% in the 2 × 1013 vector genomes/kg cohort. The levels were maintained throughout the study. Concomitant with the increase in baseline FIX, the number of spontaneous bleeds was reduced by 53% and 70% in the 2 cohorts, respectively, even though 9 of the 10 patients entered the trial on prophylaxis and had a low bleed rate already. All but 1 patient were able to discontinue prophylaxis after the gene therapy. Although 3 patients developed mildly raised alanine aminotransferase levels and were treated with steroids, no capsid-specific T-cell responses were detected. The Miesbach et al and Nathwani et al2,3 studies used the same wild-type FIX gene cassette and liver-specific promoter, yet the current study detected no capsid-specific reactive T cells even though the vector dose used was 10-fold higher. The Miesbach et al study confirms the effectiveness and safety of gene therapy in hemophilia. Interestingly, since this phase 1/2 study was concluded, the US Food and Drug Administration has allowed UniQure, the sponsor, to use the Padua mutation in its next phase 3 clinical trial without having to repeat the phase 1/2 studies.
Two of the most frequent questions asked about gene therapy by those with hemophilia are how long the efficacy will last and can we be sure it is safe. What we have so far is good news, but the future is unknown. Efficacy and safety in humans have been maintained for at least 6 years, but only time will tell how long this will last. The AAV vectors are episomal and do not integrate, so some loss of efficacy would be expected as the transduced hepatocytes turn over. The nonintegrating nature of these vectors suggests that long-term carcinogenesis will not be an issue. None of the patients with hemophilia participating in any of the gene therapy trials in hemophilia A or B has developed inhibitors, which is reassuring.
This author may be an unrealistic optimist, but he believes that in the long term the major benefit of gene therapy will be seen in resource-poor countries with little access to concentrates rather than areas where concentrates are plentiful and those with hemophilia have a reasonably good quality of life already.
Conflict-of-interest disclosure: The author declares no competing financial interests.