In this issue of Blood, Guilliams et al demonstrate that white matter is chronically hypoxic in sickle cell disease and that transfusions acutely lower the volume of brain tissue at risk for stroke.1
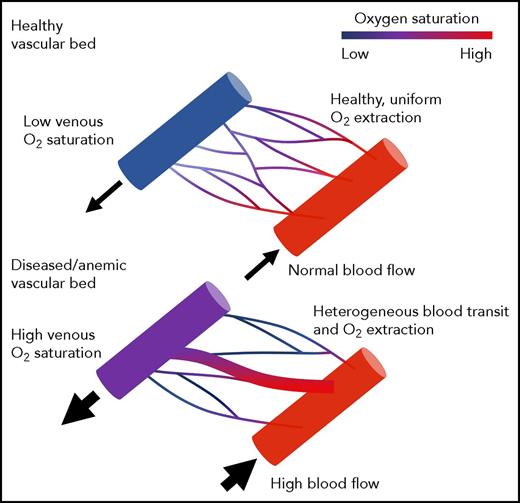
Schematic illustrating normal oxygen delivery to tissues through a healthy microvasculature (top). Slow, evenly distributed blood flow facilitates effective oxygen transfer. In this scenario, tissue OEF mirrors the oxygen difference between the artery and vein. Schematic illustrating impaired oxygen delivery in regions with vascular pruning and inhomogenous vascular transit time (bottom). Fast flow through low-resistance channels leads to high nonnutritive perfusion and ineffective oxygen unloading. The tissues are hypoxic (high OEF), even though the oxygen difference between the artery and the vein is small. Figure provided by Adam Bush.
Schematic illustrating normal oxygen delivery to tissues through a healthy microvasculature (top). Slow, evenly distributed blood flow facilitates effective oxygen transfer. In this scenario, tissue OEF mirrors the oxygen difference between the artery and vein. Schematic illustrating impaired oxygen delivery in regions with vascular pruning and inhomogenous vascular transit time (bottom). Fast flow through low-resistance channels leads to high nonnutritive perfusion and ineffective oxygen unloading. The tissues are hypoxic (high OEF), even though the oxygen difference between the artery and the vein is small. Figure provided by Adam Bush.
In the past 2 decades, regular transcranial Doppler screening, chronic transfusion, and liberal hydroxyurea utilization have dramatically reduced cerebral vasculopathy and childhood ischemic stroke. However, the mechanisms by which transfusions affect stroke risk are largely unknown. The authors use a novel magnetic resonance imaging (MRI) technique known as asymmetric spin echo (ASE) to probe brain oxygenation in 3 cohorts of patients with sickle cell disease (SCD). ASE exploits the different magnetic properties of oxygenated and deoxygenated hemoglobin to provide an estimate of regional tissue oxygen extraction fraction (OEF). OEF represents the percentage of available oxygen used by the brain and is a marker of metabolic stress. In their study, nontransfused SCD patients had markedly increased OEF and cerebral blood flow (CBF) compared with control subjects. Chronically transfused SCD patients had CBF and OEF values intermediate to those of nontransfused SCD patients and controls. More importantly, pairwise analysis showed that CBF and OEF acutely decreased with transfusion. Furthermore, hemoglobin level, but not hemoglobin S percentage, predicted CBF and OEF, suggesting that anemia severity was more important than intrinsic red cell properties.
The observed differences in OEF with transfusion were relatively small, and some might question their clinical relevance. However, the authors demonstrate a striking colocalization between brain regions exhibiting increased oxygen extraction and regions at risk of stroke. The ability to increase oxygen extraction is the brain’s fastest and most robust mechanism to buffer acute fluctuations in oxygen delivery, making resting OEF a logical predictor of future stroke risk. Longitudinal studies will be required to validate this hypothesis and define risk thresholds, but regardless of the OEF threshold chosen by the authors (36%, 38%, or 40%), transfusion reduced the volume of brain at risk by 50%. This finding supports the results of the Silent Ischemia Trial, in which chronic transfusion therapy reduced new stroke frequency by 50%.2 Thus, even relatively modest improvement in global OEF translates to significant differences in stroke risk.
The strong association between brain OEF and hemoglobin is somewhat puzzling. In the presence of anemia, the brain increases its blood flow to compensate for the decreased oxygen-binding capacity3,4 to preserve the brain’s resting oxygen delivery.3 The vasodilatory reserve of the brain is usually not exceeded until it reaches 160 mL/100 g per minute,4 which is far greater than the brain blood flow of most of the patients in this study. Nevertheless, the apparent lowering of tissue saturation observed with ASE in the present study is nearly identical to the hemoglobin dependence of tissue oxygenation observed using near-infrared spectroscopy, which uses light to perform similar tissue oxygen saturation estimates.5 Thus, the brain tissue remains ischemic despite preserved arterial oxygen delivery.
This apparent paradox can be resolved by considering the role played by the healthy microvasculature in translating whole-brain oxygen delivery into tissue oxygen delivery (see figure, top). Oxygen-rich blood perfuses tissue evenly through a rich, redundant capillary network. Upstream arteriolar tone responds to local oxygen consumption to ensure efficient oxygen supply–demand matching, as when the lung balances ventilation and perfusion. However, supply–demand matching may be grossly disrupted in diseased microvascular beds (see figure, bottom). When the microvasculature is pruned, low-resistance pathways exhibit excessive flow with little oxygen extraction, resulting in decreased global oxygen extraction and poor tissue oxygen delivery. The torrential CBF observed in SCD patients further impairs oxygen unloading to the tissues by shortening the arteriovenous transit times and limiting oxygen diffusion. Such a disconnect between global and local oxygen delivery is commonly observed in arteriovenous malformations, but recent work suggests that functional arteriovenous shunting also occurs in patients with chronic anemia syndromes, including SCD.6-8
Our work using MRI oximetry of sagittal sinus blood also supports the functional shunting hypothesis. We found that children and young adults with SCD (particularly children receiving chronic transfusion therapy) exhibited inappropriately low whole-brain OEF for their degree of anemia and CBF.8 We observed a similar phenomenon in chronically transfused thalassemia major patients. Forearm venous OEF was also decreased in both cohorts.8 In contrast, Jordan and colleagues observed increased whole-brain OEF in nontransfused adults with SCD.9 However, they used an oximetry calibration based on bovine blood at hematocrits between 35% and 55%, whereas we used a calibration based on sickle cell blood at native hematocrits,8 making it difficult to compare work between our 2 laboratories; physiological factors such as differences in patient age, genotype, hydroxyurea use, and transfusion status may also contribute to the observed differences.
Altogether, the present work and accumulating evidence from other MRI imaging modalities are starting to provide the physiological links between anemia and stroke risk. CBF is raised in anemic patients to preserve global brain oxygen delivery.3,4 Increased resting flow further shortens capillary transit times, impairing oxygen unloading. In addition, high resting CBF leaves anemic patients with an impaired cerebrovascular flow reserve. Thus, transient reduction in oxygen saturation or hemoglobin that occurs naturally (sleep apnea, parvovirus infection, splenic sequestration) does not trigger sufficient flow recruitment to protect vulnerable brain tissue.10 Deep white matter structures located in watershed areas between vascular territories have the greatest OEF at rest, leaving them vulnerable for these acute-on-chronic drops in tissue oxygen delivery.1 Current transfusion strategies decrease, but do not eliminate, the volumes of brain tissue at risk.1 Importantly, hemoglobin appears to be the strongest predictor of CBF and OEF in this report and across all studies, potentially calling into question current transfusion practice guidelines that only consider posttransfusion hemoglobin S levels. ASE and other advanced neurovascular imaging techniques will be essential to determine the optimal hemoglobin level for cerebral protection in SCD patients and the role of hemoglobin S in cerebrovascular toxicity.
Conflict-of-interest disclosure: J.C.W. serves as an MRI imaging consultant for ApoPharma, BiomedInformatics, Celgene, and WorldCare Clinical. He receives research support in-kind from Philips Healthcare. He has grant support from the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases.