TO THE EDITOR:
Acute promyelocytic leukemia (APL) has now become a highly curable disease using an all-trans retinoic acid (ATRA) and arsenic trioxide (ATO)–based treatment. The practice-changing study APL0406 reported by Lo-Coco et al demonstrated that non–high-risk APL patients, defined by a presenting white blood cell (WBC) count ≤10 × 109/L, can be cured using only ATRA and ATO without chemotherapy.1,2 We also found similar results using a totally oral arsenic and ATRA chemotherapy-free outpatient protocol (outpatient model) for non–high-risk APL.3
For high-risk APL patients (WBC count >10 × 109/L), attempts at minimizing chemotherapy have proven feasible using ATRA, ATO, and gemtuzumab ozogamicin (GO) or idarubicin with an overall survival (OS) of 65% to 87%.4-6 Because of the lack of availability of GO outside of Europe, the necessity of ATO infusions being provided in hospital settings, and relatively inferior outcomes, a novel treatment of high-risk cases is warranted. Therefore, we attempt to extend our outpatient model to high-risk APL patients.
From April 2014 through September 2016, we conducted a single-center cohort study to evaluate the efficacy and safety of oral arsenic and ATRA for high-risk APL patients (protocol provided in the supplemental Material, available on the Blood Web site). Twenty patients received oral arsenic realgar-indigo naturalis equation (60 mg/kg daily in an oral divided dose) and ATRA (25 mg/m2 daily in an oral divided dose) as induction therapy until achieving a hematologic complete remission (CR). For patients with a WBC count of (10-20) × 109/L before treatment, only hydroxyurea (3.0 g daily in an oral divided dose) was used from the first day until a WBC <10 × 109/L was achieved. For patients with a WBC count >20 × 109/L before treatment, hydroxyurea (3.0 g daily in an oral divided dose) and cytarabine (200 mg daily in an IV dose) were used from the first day to diminish the burden of leukemia until a WBC <10 × 109/L was achieved. The definition of differentiation syndrome and its moderate and severe forms were according to a previous report.7 No prophylactic drug for differentiation syndrome was administered during the induction therapy. At the earliest manifestations of suspected differentiation syndrome, prednisone (0.5 mg/kg daily) or dexamethasone (10 mg daily in an IV dose) was administered until the disappearance of signs and symptoms for a minimum of 3 days.
The consolidation therapy included realgar-Indigo naturalis formula (60 mg/kg daily in an oral divided dose) in a 4-week-on and 4-week-off regimen for 4 cycles and ATRA (25 mg/m2 daily in an oral divided dose) in a 2-week-on and 2-week-off regimen for 7 cycles. The primary endpoint was the complete molecular response (CMR) postconsolidation, defined as the absence of detectable PML-RARA transcripts using ABL as an internal control by quantitative polymerase chain reaction. Secondary endpoints included CR, event-free survival (EFS), OS, safety, and medical costs. The median follow-up was 33 months (range 16-45 months) by January 2018.
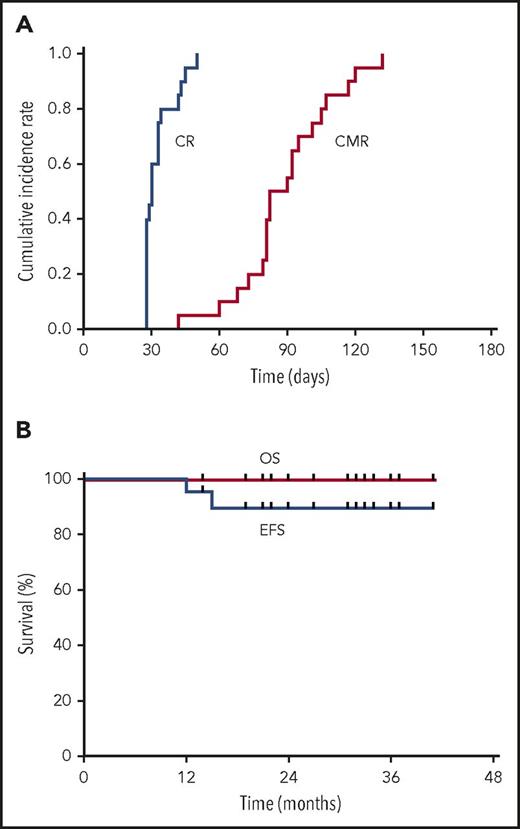
All 20 patients achieved a CR after a median time of 30 days (range, 28-50 days) (supplemental Table 1). All 20 patients received hydroxyurea, including 16 patients who received cytarabine simultaneously (supplemental Table 1). The median time of cytarabine administration was 6 days (3-14 days). The CMR rate was 85% and 100% at 3 and 6 months (Figure 1A), respectively. Grade 3 to 4 liver adverse events and differentiation syndrome occurred in 0 and 7 patients during induction (Table 1). No hematological relapse occurred. Two patients had a molecular relapse at 12 and 15 months and achieved a CMR again and maintained a continuous CR until now after receiving the same protocol mentioned above without stem cell transplantation (supplemental Table 1). The 3-year estimated OS and EFS are 100% and 89.4% (95% confidence interval, 75.49% to 100%) (Figure 1B), respectively. The total hospital time was 25 days (4-37 days) per patient. All (100%, 20/20) of the patients completed the postremission therapy on an outpatient basis without hospitalization. The median of total medical costs was $7540 ($5490-$26 530). Patients resumed their usual lifestyle during postremission therapy and thereafter.
The cumulative incidence rate of hematologic CR and CMR (A), and EFS and OS (B) for high-risk APL patients treated with oral arsenic and ATRA.
The cumulative incidence rate of hematologic CR and CMR (A), and EFS and OS (B) for high-risk APL patients treated with oral arsenic and ATRA.
The last battle for a cure for all APL patients lies in the high-risk patients. Iland et al added ATO to an ATRA plus idarubicin regimen followed by consolidation with ATRA + ATO, which achieved a 5-year OS of 87%.6 Ravandi et al demonstrated the feasibility and long-term efficacy of a chemotherapy-free strategy of ATRA + ATO + GO in a 1-arm study,5 which was confirmed by a randomized trial AML17.4 The elimination of traditional cytotoxic chemotherapy clearly has potential advantages to minimize the risk of early death and long-term side effects. Owing to the use of oral arsenic, the potential for this outpatient treatment model is likely to become a reality in high-risk APL, similar to that in non–high-risk patients.3
In summary, our study, which employed a completely oral, chemotherapy-free outpatient postremission treatment of high-risk APL, proved to be effective, convenient, and cost saving. This approach exemplifies an ideal model for the treatment of high-risk APL. However, strict clinical and laboratory monitoring of these patients for long-term outcomes is warranted.
The online version of this article contains a data supplement.
Acknowledgments
The authors are grateful to Jiong Hu (Shanghai Institute of Hematology, Shanghai Jiao Tong University School of Medicine) for helpful suggestions on the study design and manuscript, and Xiao-Jun Huang (Peking University People’s Hospital) for kindly supporting the study.
This work was supported by grants from the National Key R&D Program of China (2016YFE0202800, 2016YFC0902800) and the National Natural Science Foundation of China (81570128).
Authorship
Contribution: H.-H.Z. and Y.-R.L. had full access to all of the data and take responsibility for the data integrity and analysis; J.-S.J. reviewed the manuscript and contributed to modifications in content; Y.-Z.Q. and X.-S.Z. contributed to molecular analysis; Y.-Y.L. performed cytogenetic analysis; and all authors contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hong-Hu Zhu, No. 11 Xizhimen South St, Beijing, China; e-mail: zhuhhdoc@163.com.