Abstract
Introduction - Various pre-transplantation factors help predict outcomes in patients (pts) with myelofibrosis (MF) undergoing allogeneic stem cell transplantation (HCT). However, early post-HCT characteristics have not been correlated with outcomes. Our study aims at identifying day +100 characteristics that are indicative of poorer outcomes.
Methods - Pts undergoing HCT for MF at the 3 sites of Mayo Clinic in Rochester, Arizona and Florida, between January 2005 and August 2016, were retrospectively reviewed for collecting baseline, disease, transplantation and outcome data. Day +100 data was collected as available between day +80 through day +120. Day +100 characteristics included molecular status, WHO grade of bone marrow fibrosis, spleen size, red blood cell (RBC) transfusion dependence and platelet transfusion dependence. Transfusion dependence was defined as RBC or platelet transfusions requirements of 2 units in a 4 week period. Since there was limited data on donor chimerism and molecular marker status over the years, we used a variable called "molecular status" to reflect on the molecular response after HCT. Molecular status was defined as favorable if either 100% donor chimerism in CD33 fraction from blood or molecular marker negativity was known and as unfavorable if donor chimerism <100% or molecular marker positivity was known at day +100. Survival analysis was conducted using Kaplan-Meier (K-M) survival curve and a series of univariate and multivariate Cox proportional hazard regression models. Patient characteristics measured at day +100 along with age at HCT, gender, Dynamic International Prognostic Scoring System (DIPSS)-Plus score, conditioning regimen (myeloablative vs reduced intensity) and donor source (related vs unrelated) were used to examine group differences in K-M curves and to predict overall survival in the regression models.
Results -
Baseline/ Transplantation characteristics : A total of 87 pts underwent HCT for MF, of which, 79 were included in our study. Eight pts who died within 80 days from HCT were not included in the analysis. Median age at HCT was 58 years (range, 19-73 years). Forty seven (60%) were men and MF was primary in 54 (68%) pts. DIPSS-Plus score was high in 34 (43%), intermediate-2 in 40 (51%) and intermediate-1 in 5 (6%) pts. Conditioning regimen was myeloablative in 14 (18%) and reduced intensity in 65 (82%). Anti-thymocyte globulin was used in 35 (44%) pts. Donor source was related in 39 (49%) and unrelated in 40 (51%) pts. Of the related donors, 2 were haploidentical donor HCTs. JAK2 mutation was positive in 41 (54%) out of 76, CALR was positive in 8 (38%) out of 21 and MPL was positive in 4 (13%) out of 31 pts, in whom the status was available. Forty three (57%) pts were ABO compatible while 19 (25%) had minor ABO incompatibility, 12 (16%) had minor incompatibility and 1 (1%) had bidirectional ABO incompatibility. Ten (13%) pts had undergone splenectomy prior to HCT.
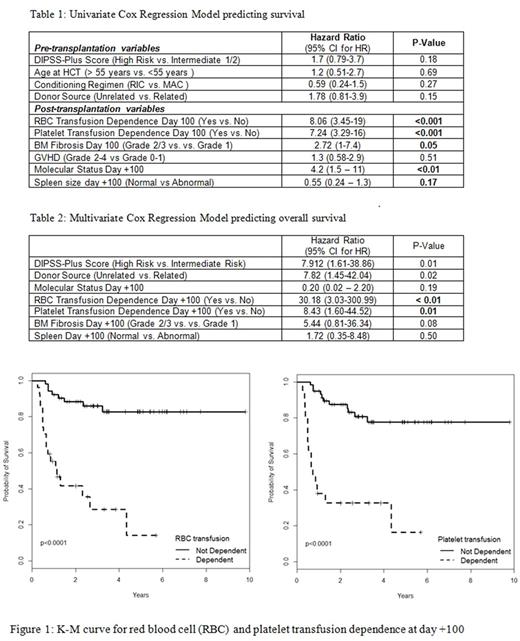
Survival analysis : Twenty-six (33%) pts had died at the time of this study. Cause of death was HCT-related in 19 (73%) and disease relapse/ progression in 7 (27%) pts. RBC transfusion dependence, platelet transfusion dependence and unfavorable molecular status at day +100 were associated with increased risk of death in the univariate model (Table 1). The significant and nearly significant day +100 predictors from the univariate models were included in the multivariate model (table 2) along with pre-transplant characteristics. RBC and platelet transfusion dependence were significant predictors associated with poorer overall survival in the multivariate analysis (hazard ratios 30.18 and 8.43, respectively). Bone marrow fibrosis of grade ≥ 2 showed a trend towards poorer OS in the univariate analysis, but was not statistically significant in the multivariate model (hazard ratio 5.44, p= 0.08).
Conclusions - In this exploratory analysis of day +100 variables for predicting long-term outcomes in pts undergoing HCT for MF, we report a strong relationship between RBC/ platelet transfusion dependence at day +100 and increased risk of death (figure 1). The mechanism of this relationship is not clear. These pts could, perhaps, benefit from close monitoring or early intervention. Given the scope of the current study, larger studies are warranted to further investigate these findings and identify the cause of this association.
Khera: Novartis: Consultancy. Mesa: Novartis Pharmaceuticals Corporation: Consultancy; Gilead Sciences, Inc.: Research Funding; Incyte Corporation: Research Funding; Ariad: Consultancy; Promedico: Research Funding; Celgene Corporation: Research Funding; CTI BioPharma Corp.: Research Funding; Galena Biopharma, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.