Abstract
INTRODUCTION: The effects of ibrutinib treatment on the natural history of autoimmune cytopenias (AIC) among chronic lymphocytic leukemia (CLL) patients (pts) treated on clinical trials suggests: i) a low rate of treatment-emergent (TE) AIC (~2%, Rogers, Leukemia 2016; Montillo, BCJ 2017) and ii) improved control of pre-existing AIC at the time ibrutinib is initiated with the ability to discontinue immunosuppressive therapies (~69%, Vitale, Haematologica 2016). Confirmation of these findings in CLL pts treated in routine clinical practice requires further investigation.
METHODS: Between 4/2012 and 1/2017, 286 CLL pts treated with ibrutinib outside the context of a clinical trial were seen in the Division of Hematology at Mayo Clinic. The CLL Database and medical records of these pts were reviewed for details of past history of AIC and TE AIC; the diagnosis of AIC was determined by the treating physician. Cox proportional hazards regression models were used to estimate hazard ratios and 95% confidence intervals for the association of history of TE AIC with progression-free (PFS) and overall (OS) survival. Time-dependent Cox regression models were used to look at the association of TE AIC with PFS and OS. Fisher's Exact test was used to test for the association of clinical variables with TE.
RESULTS: Among the 286 pts, 242 (85%) pts were treated for relapsed CLL and 44 (15%) pts for previously untreated CLL. The median age at ibrutinib start was 68 years, and 72% were males. Twenty-two percent had TP53 disruption (either del17p or TP53 mutation) and 78% had unmutated IGHV genes. We identified 37/286 (13%) pts with a history of AIC prior to ibrutinib start, including 15 pts with autoimmune hemolytic anemia (AIHA), 11 pts with immune thrombocytopenia (ITP), 5 pts with Evans syndrome, 3 pts with pure red cell aplasia (PRCA), 2 pts with autoimmune neutropenia, and 1 pt with aplastic anemia.
Eleven (4%) pts developed TE AIC (including two pts with past history of AIC who developed a different AIC after ibrutinib therapy) including: AIHA (n=4), ITP (n=4), PRCA (n=1), aplastic anemia (n=1), and autoimmune neutropenia (n=1). This occurred after a median of 30 days following the initiation of ibrutinib (range, 8-325 days). Eight pts were able to continue ibrutinib after either temporarily holding ibrutinib (n=6), ibrutinib dose reduction (n=1), or no modification to ibrutinib therapy (n=1). Three of the 11 pts (ITP n=2, aplastic anemia n=1) permanently discontinued ibrutinib as a result of the AIC.
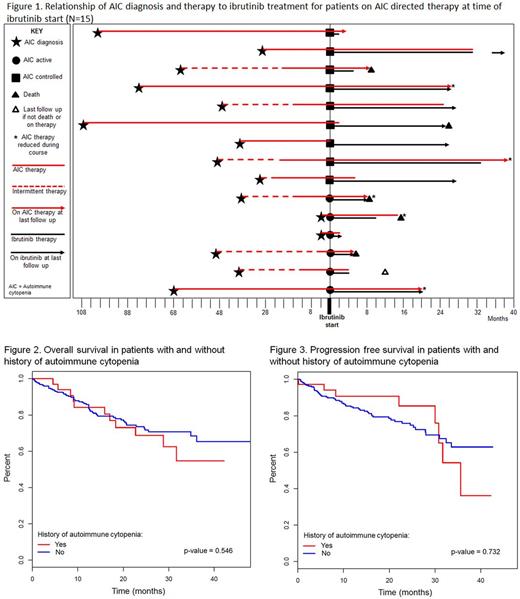
Twenty-two of the 37 pts with a prior history of AIC had a remote history of AIC and were not on any AIC treatment and 15/37 pts were receiving AIC directed therapy at ibrutinib start. Among the 15 pts who were on AIC directed therapy at the time of ibrutinib initiation, 6 pts had uncontrolled disease, for which they were receiving ≥20 mg prednisone daily, and 9 pts had controlled AIC on therapy consisting of <20 mg of prednisone daily or equivalent. Five (33%) of the 15 pts on therapy were able to discontinue AIC therapy at a median of 2 months (range, 0-25), an additional 5 (33%) pts were able to reduce the dose and schedule of AIC therapy, and 3 (20%) pts continued AIC therapy with no changes. Two (14%) pts not previously on rituximab were started on the therapy due to continued steroid dependence and inability to taper off steroids. None of these 15 pts had worsening of their underlying AIC as a consequence of initiating ibrutinib therapy. See Figure 1 for temporal relationship of ibrutinib treatment.
The median OS and PFS from time of ibrutinib start was not significantly different between pts with a history of AIC and those with no history of AIC (Figures 2 and 3). There was no statistically significant association between age, gender, TP53 disruption, IGHV mutation status, and history of AIC with development of TE or recurrent AIC (p-values all > 0.05). The PFS and OS were not significantly different among pts with TE AIC compared to those pts who did not develop TE AIC.
CONCLUSION: In this large retrospective cohort of ibrutinib treated CLL pts outside the context of clinical trials, we found that 1 out of every 25 pts developed TE AIC. Nonetheless, ~75% pts were still able to continue ibrutinib treatment. Improved control of AIC with discontinuation or de-escalation of AIC treatments in ~67% of pts on AIC directed therapy at ibrutinib start supports previous findings. We did not identify any risk factors for the development of TE AIC in our study.
Ding: Merck: Research Funding. Kay: Gilead: Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Tolero Corporation: Research Funding. Shanafelt: Genentech: Research Funding; GlaxoSmithKline: Research Funding; Celgene: Research Funding; AbbVie: Research Funding; Hospira: Research Funding; Jannsen: Research Funding; Pharmacyclics: Research Funding. Parikh: AstraZeneca: Honoraria; Pharmacyclics: Honoraria; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.