Abstract
Introduction
Hematopoietic stem cell transplant (HSCT) after induction/consolidation therapy with standard chemotherapy or targeted therapies, such as blinatumomab or inotuzumab (Kantarjian H, et al. N Engl J Med . 2016;375:740-53), is considered the only treatment option with curative intent for patients with relapsed/refractory Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia (r/r Ph- B-precursor ALL). A recent randomized phase 3 study of blinatumomab, a bispecific T-cell engager antibody construct, in patients with r/r Ph- B-precursor ALL, included a maintenance treatment phase to allow patients in hematologic remission to continue blinatumomab (Kantarjian H, et al. N Engl J Med . 2017;376:836-847). In this exploratory analysis, we examined overall survival (OS) for patients who received blinatumomab maintenance therapy and compared outcomes with data pooled from patients who did not receive maintenance therapy in the phase 3 and phase 2 (Topp MS, et al. Lancet Oncol . 2015;16:57-66) blinatumomab trials.
Methods
Adult patients (≥18 years) in this phase 3 trial were randomized to either blinatumomab or standard of care (SOC) chemotherapy. Patients randomized to blinatumomab received this treatment by continuous intravenous infusion (4 weeks on, 2 weeks off; 9 μg/day on days 1-7 of Cycle 1 and 28 μg/day thereafter) during induction and consolidation cycles. Patients could receive HSCT at any time after cycle 1. Patients who received blinatumomab, and had bone marrow blasts <5% after two induction and three consolidation cycles of blinatumomab, were eligible for blinatumomab maintenance, administered for up to an additional 12 months (4 weeks on therapy, 8 weeks off). Maintenance therapy was discontinued in case of transition to HSCT, investigator discretion, toxicity, relapse, or use of protocol-excluded medications. The cumulative probability of OS over time was estimated using the Simon-Makuch method and the effect of maintenance was analyzed using the Mantel-Byar method. OS was measured from the start of maintenance therapy for patients who were in remission until the start of Cycle 6 (for those who received maintenance) or at Day 211 (landmark date for those who did not receive maintenance). Data from the phase 3 study and an earlier phase 2 study in which maintenance was not offered were pooled to increase the sample size for this OS analysis.
Results
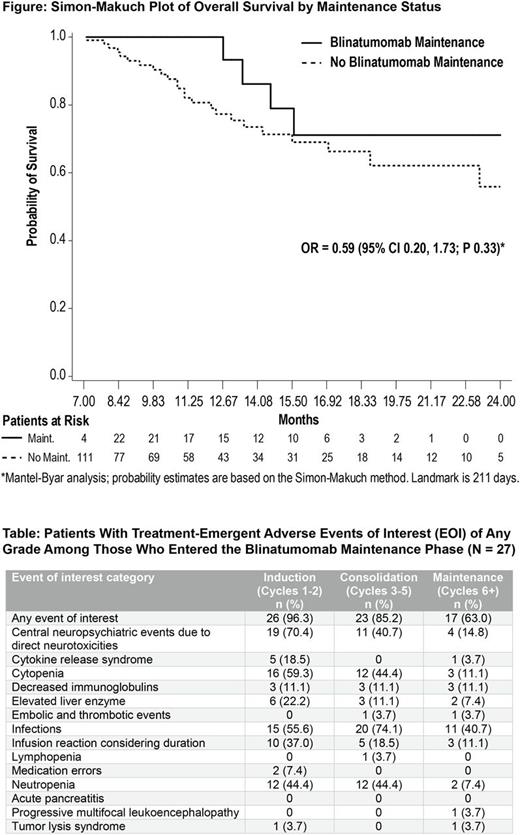
As of the primary analysis cutoff, 119 of 271 (43.9%) patients receiving blinatumomab achieved a best response of CR/CRh/CRi within two treatment cycles, and 27 patients continued to the blinatumomab maintenance phase. Three patients completed the maintenance phase, 4 transitioned to HSCT, and one discontinued due to adverse events (AE). Twenty-one (77.8%) patients had a best response of CR during maintenance, 1 (3.7%) patient each had CRh or blast free hypoplastic or aplastic bone marrow, and 2 (7.4%) patients each had hematologic relapse or were not evaluable. The Mantel-Byar analysis demonstrated a relative OR for OS of 0.59 (95% CI, 0.20 - 1.73) P = 0.33 comparing maintenance vs. no maintenance (Figure), representing a 41% reduction in the risk of death associated with maintenance therapy, though not statistically significant. Among patients receiving blinatumomab who entered the maintenance phase, the incidence of adverse events of interest generally was less compared with the incidence during the induction or consolidation phases (Table).
Conclusions
In r/r ALL patients who achieved a hematologic response following blinatumomab induction/consolidation, high response rates with blinatumomab maintenance therapy (beyond cycle 5) were observed, with longer OS compared with patients receiving no maintenance. During maintenance with blinatumomab, no new safety concerns were identified. A lower incidence of adverse events of interest was seen during the maintenance phase, compared with events recorded during the induction/consolidation phases of this, and prior blinatumomab studies.
Research supported by Amgen Inc.
Rambaldi: Novartis, Roche/Genentech, Amgen, Italfarmaco: Consultancy; Novartis, Amgen, Celgene, Sanofi: Other: Travel, Accomodations, Expenses. Rigal-Huguet: Bristol Myers-Squibb, Novartis, Pfizer, Amgen, Incyte, Jazz Pharma: Consultancy; Bristol Myers-Squibb, Novartis, Pfizer, Amgen, Incyte, Jazz Pharma: Honoraria. Nie: Amgen: Employment, Equity Ownership. Zimmerman: Amgen Inc.: Employment, Equity Ownership. Topp: Amgen: Consultancy, Honoraria, Other: Travel, Research Funding; Regeneron: Consultancy, Honoraria, Research Funding; Celgene: Other: Travel; Roche: Consultancy, Research Funding; Macrogenics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.