In this issue of Blood,Merrill et al report that stopping therapy with eculizumab in 15 adult patients with atypical hemolytic syndrome (aHUS), after remission was achieved, resulted in relapse in only 3 individuals.1
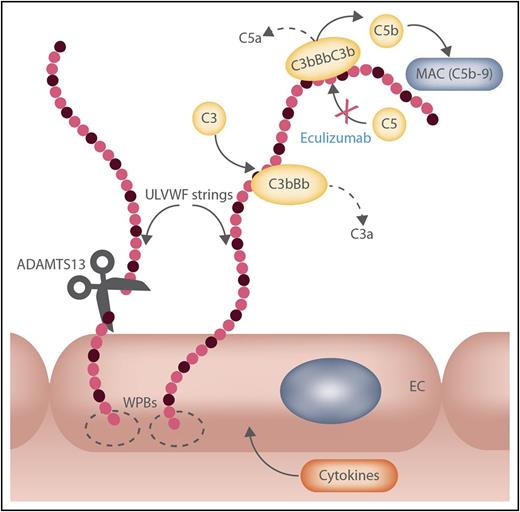
Linking von Willebrand factor (VWF) and the alternative complement pathway. Activation and amplification of the alternative complement pathway (AP) can occur on ultra-large (UL) VWF strings. The image depicts assembly of C3 convertase (C3bBb) and C5 convertase (C3bBbC3b) on endothelial cell (EC) secreted/anchored ULVWF multimeric strings, leading to the formation of copies of the membrane attack complex (MAC; C5b-9) that are potentially injurious to target (and host) cells. In individuals with ≥1 mutation/polymorphism promoting amplification of the AP (eg, by impairing the negative regulatory activity on C3 convertase by factor H, factor I, or CD46), acute aHUS episodes are likely. Normally, the VWF-cleaving protease ADAMTS13, released from EC cytoplasm, diminishes the brief time available for AP activation by the EC-secreted/anchored ULVWF strings. This proteolytic control mechanism is likely to be less effective if functional ADAMTS13 production is reduced even moderately (∼50% of normal ADAMTS13 activity is shown as cleavage of 1 of 2 ULVWF strings) and will be compromised further if the rate of ULVWF secretion/anchorage is increased by cytokines during inflammation or infection. Not shown are complement components regulating C3 convertase positively (properdin or factor P) or negatively (factors H and I and CD46) and platelet adherence to the ULVWF strings. C3b, Bb, C5b, activated C3, factor B, or C5, respectively; C3a, C5a, small cleavage products of C3 or C5; C5b-9, MAC complex composed of C5b, C6, C7, C8, and C9; WPB, Weibel-Palade body. Original figure prepared by Nancy A. Turner, Rice University. Professional illustration by Somersault18:24.
Linking von Willebrand factor (VWF) and the alternative complement pathway. Activation and amplification of the alternative complement pathway (AP) can occur on ultra-large (UL) VWF strings. The image depicts assembly of C3 convertase (C3bBb) and C5 convertase (C3bBbC3b) on endothelial cell (EC) secreted/anchored ULVWF multimeric strings, leading to the formation of copies of the membrane attack complex (MAC; C5b-9) that are potentially injurious to target (and host) cells. In individuals with ≥1 mutation/polymorphism promoting amplification of the AP (eg, by impairing the negative regulatory activity on C3 convertase by factor H, factor I, or CD46), acute aHUS episodes are likely. Normally, the VWF-cleaving protease ADAMTS13, released from EC cytoplasm, diminishes the brief time available for AP activation by the EC-secreted/anchored ULVWF strings. This proteolytic control mechanism is likely to be less effective if functional ADAMTS13 production is reduced even moderately (∼50% of normal ADAMTS13 activity is shown as cleavage of 1 of 2 ULVWF strings) and will be compromised further if the rate of ULVWF secretion/anchorage is increased by cytokines during inflammation or infection. Not shown are complement components regulating C3 convertase positively (properdin or factor P) or negatively (factors H and I and CD46) and platelet adherence to the ULVWF strings. C3b, Bb, C5b, activated C3, factor B, or C5, respectively; C3a, C5a, small cleavage products of C3 or C5; C5b-9, MAC complex composed of C5b, C6, C7, C8, and C9; WPB, Weibel-Palade body. Original figure prepared by Nancy A. Turner, Rice University. Professional illustration by Somersault18:24.
In addition to the observations of Merrill et al, several other articles published during 2014 to 2017 have reported that discontinuing eculizumab in patients during remission resulted in relapse in only ∼one-third of patients.2-4 Eculizumab is a monoclonal antibody directed against complement component C5 (see figure) that is effective in inducing remission in pediatric and adult patients with acute episodes of aHUS. The anti-C5 antibody is also extremely expensive and increases the risk of serious bacterial infections when administered weekly over prolonged time periods.
Sites for the direct binding, activation, and amplification of complement component C3 are present on ULVWF multimeric strings secreted from the Weibel-Palade bodies of human endothelial cells5,6 stimulated by tumor necrosis factor (TNF), interleukin-8, and several other cytokines7 and chemicals. The ULVWF strings remain anchored to the endothelial cell surface until cleaved by the VWF-cleaving protease ADAMTS13 (see figure). A reasonable supposition is that acute episodes of aHUS can be provoked by ULVWF string secretion from, and anchorage to, intensely stimulated endothelial cells, with associated alternative complement pathway (AP) activation, in individuals who have single (or multiple) heterozygous mutations promoting AP activation or amplification. AP activation/amplification on secreted/anchored ULVWF strings would be predicted to be even more likely in patients with a single (heterozygous) loss-of-function mutation in a gene for ADAMTS131 that compromises the extent of cleavage of secreted/anchored ULVWF strings.8
aHUS episodes are associated with mutations affecting ≥1 component gene in the AP that influences activation (eg, of C3 or factor B) or regulation (eg, factor H, factor I, or CD46). In the accompanying article, Merrill et al report that 11 of 13 patients with aHUS tested for genetic abnormalities had various AP-related and/or ADAMTS13 mutations. Specifically, 9 had single or multiple AP-related heterozygous mutations; in 2, the mutations were homozygous. For some of these mutations (deletion of factor H–related genes 1 and 3), the precise mechanisms causing a propensity for excessive AP activation, with presumed increased generation of C5 and the membrane attack complex (see figure), have not been defined precisely.
Three of the patients reported by Merrill et al had heterozygous mutations of ADAMTS13. The median functional activity of ADAMTS13 in all 17 patients was 60% that of normal. It will be useful in future clinical studies to include, as did Merrill et al, analysis of the ADAMTS13 gene to determine if heterozygous AP and ADAMTS13 defects in combination do indeed increase the likelihood of acute aHUS episodes.
The data of Merrill et al suggest that inflammation or infection may have precipitated relapses in the 3 patients with aHUS who discontinued eculizumab. Inflammatory events in vivo are associated with increased cytokine production. In vitro, the powerful inflammatory cytokine TNF stimulates endothelial cell secretion/anchorage of ULVWF strings plus associated AP activation.9 Subsequent clinical studies are likely to clarify whether or not discontinuation of eculizumab in patients with aHUS in remission is safe in the absence of underlying inflammation or infection.
Conflict-of-interest disclosure: The author declares no competing financial interests.