Abstract
During the last decade, the development of improved and novel approaches for the treatment of hemophilia A has expanded tremendously. These approaches include factor VIII (FVIII) with extended half-life (eg, FVIII-Fc and PEGylated FVIII), monoclonal antibodies targeting tissue factor pathway inhibitor, small interfering RNA to reduce antithrombin expression and the bispecific antibody ACE910/emicizumab. Emicizumab is a bispecific antibody recognizing both the enzyme factor IXa and the substrate factor X. By simultaneously binding enzyme and substrate, emicizumab mimics some part of the function exerted by the original cofactor, FVIII, in that it promotes colocalization of the enzyme–substrate complex. However, FVIII and the bispecific antibody are fundamentally different proteins and subject to different modes of regulation. Here, we will provide an overview of the similarities and dissimilarities between FVIII and emicizumab from a biochemical and mechanistical perspective. Such insight might be useful in the clinical decision making for those who apply emicizumab in their practice now or in the future, particularly in view of the thrombotic complications that have been reported when emicizumab is used in combination with FVIII-bypassing agents.
Introduction
Hemophilia A is an X-linked bleeding disorder caused by defects in the gene encoding factor (F)VIII affecting 1 or 2 per 10 000 male births. Treatment of hemophilia is dictated by its clinical severity and most frequently involves replacement therapy using plasma-derived or recombinant FVIII concentrates. Replacement therapy has major benefits in the clinical management of hemophilia A, and when administered prophylactically, it can significantly reduce spontaneous bleeding episodes.1,2 Nevertheless, FVIII replacement therapy has a number of limitations, including the development of neutralizing antibodies in up to 30% of patients with severe hemophilia A, rendering replacement therapy ineffective.3-5 The inhibitor rate is 5% to 13% in mildly or moderately affected patients and is associated with increased morbidity and mortality.6,7 Currently, bleeding in inhibitor patients is managed using bypassing agents that work independently of FVIII, like recombinant activated factor VII and activated prothrombin complex concentrates. In addition, immunologic tolerance to FVIII may be induced via repeated high-dose infusions of the protein. Both approaches are costly and not always successful.
To overcome limitations of FVIII replacement therapy, the search for improved FVIII variants or alternative treatment options has been intensified. This has resulted in the development of FVIII variants with extended half-life (eg, FVIII-Fc and PEGylated FVIII).8,9 Alternative approaches are aiming to restore the hemostatic balance by interfering with anticoagulant pathways. Examples hereof are concizumab, a monoclonal antibody targeting tissue factor pathway inhibitor, and fitusiran, an small interfering RNA approach to reduce expression of antithrombin.10,11 It is without doubt that also other approaches will surface in the near future.
A third variant is the bispecific antibody emizicumab (also known as ACE910) developed by Chugai Pharmaceutical that is designed to mimic (at least in part) FVIII cofactor activity.12,13 Clinical trials using emicizumab revealed hemostatic activity in hemophilia A patients with and without inhibitors, with significant reductions in annualized bleeding rates.14-16 Although the concept of this antibody is elegant, reality teaches us that the FVIII-mimicking activity of emicizumab is less straightforward than many seem to appreciate. We therefore provide an overview of the similarities and dissimilarities between FVIII and emicizumab from a biochemical and mechanistical perspective. Understanding its mode of action is relevant to its incorporation into clinical decision-making, particularly when this bispecific antibody is used in combination with other procoagulant agents.16
Functional aspects of activated FVIII in the FX–activating complex
Before going into detail about ACE910/emicizumab, it may be relevant to remember the complex mode of action of FVIII. FVIII is present in plasma as a nonactive procofactor at a concentration of ∼0.1 μg/mL. It circulates predominantly as a heterodimeric protein, consisting of a metal ion-linked heavy and light chain, tightly associated to its chaperone-protein von Willebrand factor (VWF).17-20 The 80-kDa light chain contains the a3-A3-C1-C2 domains. The heavy chain consists of the A1-a1-A2-a2-B domains and is heterogeneously sized (90-200 kDa) due to limited proteolytic processing at a number of positions in the B-domain.18,21 The a1, a2, and a3 regions represent polypeptide chains enriched in acidic amino acids needed for the sulfation of 6 tyrosine residues in these regions.22
To participate in the FX-activating complex, FVIII needs to be converted into its activated derivative, FVIIIa. Activation is mainly achieved by thrombin-mediated limited proteolysis in both the heavy chain (positions p.Arg372 and p.Arg740) and light chain (position p.Arg1689), although FXa in the presence of phospholipids or the activated FVII/tissue factor complex also possesses the capacity to activate FVIII.23-25 This activation step generates a labile heterotrimeric activation product in which the high-affinity VWF-binding site is lost because of the release of the p.Tyr1680-containing a3 fragment.23,26 Thus, FVIIIa consists of a metal ion–linked A1-a1/A3-C1-C2 dimer noncovalently associated with the A2-a2 domain. Once activated, FVIIIa is able to enhance the velocity of FXa generation by FIXa. Dependent on the experimental conditions used (eg, phospholipid concentration, phospholipid composition, protein concentrations), this enhancement may reach 103- to 106-fold.27
The formation of the FX-activating complex is a multistep process. The first step involves colocalization of the FIXa–FVIIIa complex at the phospholipid surface. FVIIIa binds to phospholipids ∼50-fold more efficiently than FIXa (apparent affinity constant [KD,app] 0.24 nM and 12 nM, respectively).28,29 Binding to the phospholipid surface limits movements of both proteins to a 2-dimensional direction, which results in a higher apparent affinity (15 nM in the absence vs 2 nM in the presence of phospholipids).30,31 This primary interaction involves an extended surface overlapping the FIXa light chain (the Gla domain and probably the epidermal growth factor (EGF)-like domains) and various regions in the FVIIIa A3, C1, and C2 domains.32 This association permits a proper positioning of the FVIIIa A2-a2 fragment toward the FIXa protease domain, favoring a secondary interaction between the latter two. This secondary interaction is highly relevant for optimal cofactor activity. For instance, the simultaneous binding of FIXa to the A2 domain and A1-a1/A3-C1-C2 dimer stabilizes the heterotrimeric structure of FVIIIa by slowing down spontaneous dissociation of the A2-a2 fragment.33 In a complementary manner, the A2 domain contributes to the stabilization and optimal orientation of the FIXa active site.34 This process involves interactions with several distinct regions in the FIXa protease domain: the 199-204 surface loop, the 256-268 insertion loop, and the 333-338 helix loop.35-37 Besides localizing FIXa to the phospholipid surface and orientating its active site, FVIIIa also functions as a molecular bridge between FIXa and its substrate, FX.38-40 Akin to the interaction with FIXa, binding of the substrate FX involves an initial step involving the FVIII light chain (KD,app 0.17-0.37 μM) and a secondary step allowing a low-affinity interaction (KD,app 1-3 μM) between the acidic region a1 and the FX protease domain.39,40 This cofactor–substrate interaction brings enzyme and substrate in close proximity, which from an energetic perspective will further favor the generation of FXa.
ACE910/emicizumab: development and mode of function
ACE910/emicizumab is a bispecific antibody containing 2 different antigen-binding fragments, one recognizing FIX/FIXa and the other recognizing FX/FXa. To arrive to this bispecific antibody, a total of 200 monoclonal antibodies against each component were generated in mice, rats, and rabbits. Of the 40 000 possible combinations, 94 (0.24%) displayed at least some enhancement of FXa generation, and after matching for light-chain and framework sequences, one candidate (BS15L) was selected for further optimization.41 BS15L consisted of a rat anti-FIX variable heavy chain and a mouse anti-FX variable heavy chain grafted on a human immunoglobulin G4 framework, combined with a mouse/rat hybrid variable light-chain sequence grafted on a human κ chain. Optimization steps were performed via site-directed mutagenesis of specific amino acids and included antibody humanization, improvement of FXa generation enhancement, improvement of pharmacokinetic properties, changing isoelectic points of the individual arms to facilitate purification, and improvement of solubility.41 Following each step of improvement, the potential effects of the mutational changes on antibody activity and immunogenicity were verified. These steps finally resulted in the antibody designated ACE910, now known as emicizumab.
Compared with FVIII, the mode of action of emicizumab is relatively straightforward. By interacting with both FIXa and FX, it is able to bring enzyme and substrate in close proximity, thereby facilitating FIXa-mediated FX activation.12,41 In chromogenic assays using purified FIXa and synthetic phospholipid vesicles, generation of FX is enhanced several-fold upon the addition of ACE910/emicizumab, confirming that the antibody is able to take over at least some of the tasks executed by FVIIIa. In this particular assay, the catalytic efficiency (kcat/Km) by which ACE910/emicizumab enhances FXa generation appears to be ∼10-fold reduced compared with FVIIIa.12 However, this catalytic efficiency has been determined in the absence of FIX, which may compete with FIXa for binding to the antibody. Interestingly, no FXa is generated in the absence of phospholipids, suggesting that positioning of FIXa and FX at the phospholipid surface is still essential for a proper alignment of enzyme and substrate.
Similarities and dissimilarities between FVIII and ACE910/emicizumab
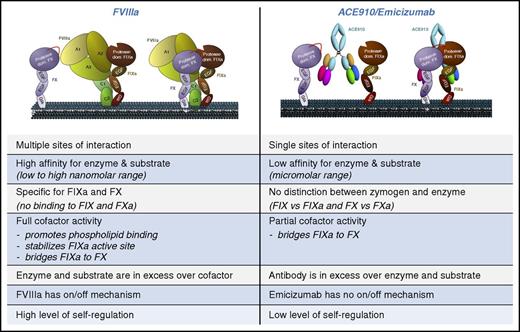
Having established that both FVIIIa and ACE910/emicizumab are able to enhance FXa generation, it is of interest to make a step-by-step comparison of the molecular properties of both proteins (Figure 1).
Schematic representation of how FVIIIa and ACE910/emicizumab promote FXa generation. Comparison of the various differences in the interactions with enzyme and substrate between FVIIIa and ACE910/emicizumab. In the left panel, FVIIIa is bound to the phospholipid surface only by its C2 domain.53,54 Other models predict that also the C1 domain may contribute directly to phospholipid binding (eg, see Meems et al55 ).
Schematic representation of how FVIIIa and ACE910/emicizumab promote FXa generation. Comparison of the various differences in the interactions with enzyme and substrate between FVIIIa and ACE910/emicizumab. In the left panel, FVIIIa is bound to the phospholipid surface only by its C2 domain.53,54 Other models predict that also the C1 domain may contribute directly to phospholipid binding (eg, see Meems et al55 ).
Interactions with enzyme and substrate
Sites of interaction. The topology by which FVIIIa and ACE910/emicizumab interact with the enzyme and substrate is dissimilar. FVIIIa/FIXa and FVIIIa/FX interactions both involve several distinct binding sites located in both heavy and light chains of each protein. In contrast, ACE910/emicizumab binds to a single site within the FIX(a) EGF1-like domain and within the FX(a) EGF2-like domain.42
Affinity of interaction. There is a substantial difference in the affinity by which FVIIIa and ACE910/emicizumab bind to enzyme and substrate. In the absence of phospholipids, FVIIIa binds FIXa with an affinity of ∼15 nM, whereas ACE910/emicizumab has a much lower affinity for FIXa, with a KD of 1.5 μM.30,42 Similarly, the affinity of FVIIIa for FX appears to be ∼0.3 μM, whereas the interaction between the bispecific antibody and FX is defined by a sixfold weaker KD of 1.8 μM.40,42 Such differences in affinity are relevant, since they are a major determinant of how much complex may be formed. Assuming plasma concentrations for FIX and FX of 90 nM and 135 nM, respectively, a bell-shape curve describes the formation of a ternary FIX–FX–antibody complex when varying antibody levels.42 At average therapeutic antibody levels of 55 μg/mL (0.37 μM), ∼0.8 nM ternary complex will be formed, whereas optimal complex formation occurs at antibody levels of 265 μg/mL (1.8 μM; producing 1.7 nM complex). At higher antibody concentrations, ternary complex formation will diminish because FIX and FX will start binding to separate antibody molecules.
Noteworthy, in patients who receive FIX- or FX-containing concentrates, such as FEIBA, the stoichiometry of complex formation may be influenced by the increased concentrations of FIX and FX. For instance, a twofold increase in FX concentration enhances ternary complex formation approximately twofold.42
Stoichiometry of complex formation. As stated above, the plasma concentrations of FIX and FX are ∼90 nM and 135 nM, respectively, whereas the concentration of FVIII is 0.4 nM. This implies that in the FX activating complex, the activity of the complex is limited by the amount of FVIIIa generated during the procoagulant response. Emicizumab levels are targeted to be 0.37 μM during prophylactic treatment.16 Consequently, the factor that is limiting FXa generation is no longer FVIIIa but the amount of FIXa that is being generated. This notion is important not only for the establishment of optimal treatment conditions but also for the interpretation of the procoagulant activity of ACE910/emicizumab in the different activity assays.
Mechanistic aspects
Recognition zymogen vs enzyme. FIX and FVIII require several proteolytic steps before complex assembly takes place. Proteolysis is needed to liberate FVIII from VWF and to convert FIX and FVIII into their active conformation. Thus, cofactor activation represents an “on” switch for the FX activating complex. The distinction between precursor and activated derivatives is absent for ACE910/emicizumab, because the antibody displays similar affinity for FIX and FIXa (1.6 μM and 1.5 μM, respectively) and for FX and FXa (1.9 μM and 1.0 μM, respectively).42 The notion that the bispecific antibody recognizes both FIX and FIXa implies that the antibody is always in the “on” mode as soon as FIXa is present. This notion is of relevance when ACE910/emicizumab is used in combination with FIXa-containing concentrates. For example, it has previously been reported that FEIBA contains substantial concentrations of FIXa (1.0-1.2 μg/mL, corresponding to 22-27 nM, approximately one-quarter of the normal FIX plasma concentration).43,44 Consequently, the presence of increased FIXa levels will result in accelerated FXa generation.
Mimicking FVIIIa functions. FVIIIa stimulates FXa generation in three distinct ways: by promoting localization of FIXa (and probably also FX) at the phospholipid surface, via proper orientation and stabilization of the FIXa active site, and by bringing FIXa and FX in close proximity. In contrast to this multiple mode of action of FVIIIa, ACE910/emicizumab is unable to enhance phospholipid binding of the individual enzyme and substrate, nor does it modulate the orientation or stability of the FIXa active site. Its function is limited to bridging enzyme and substrate in a side-by-side manner. It is surprising therefore that in kinetic studies using purified proteins, the apparent Km value was 10-fold lower in the ACE910/emicizumab-catalyzed reaction than the FVIIIa-catalyzed reaction (2.5 nM vs 25 nM, respectively).12 This may perhaps be explained by the notion that the rate of FXa generation is directly proportional to the amount of FX that bind to the phospholipid surface.45 Given the limited number of available binding sites for FX when using phospholipids at a concentration of 20 μM (40 nmol/20 μmol phospholipid),29 the presence or absence of FVIIIa (used at a concentration of 30 nM) is thus an important determinant of how many binding sites are left for FX. Emicizumab will not occupy binding sites at the phospholipid surface, allowing more FX to bind than when FVIIIa is present, while using similar FX concentrations in both assays. This will artificially result in a lower apparent Km value when using emicizumab vs FVIIIa.
Location of FXa generation. Many of the in vitro assays use artificial phospholipids, whereas the tenase complex is believed to mainly assemble at the surface of activated platelets in vivo.46,47 Thrombin-activated platelets expose limited phosphatidyl-serine, resulting in an outer membrane composition of 1% to 4% phosphatidyl-serine,48 which is much lower than used in purified phospholipid vesicles (commonly 10% to 40%). This low phosphatidyl-serine exposure is insufficient to promote efficient tenase activity. Indeed, Gilbert et al48 have recently demonstrated that fibrin present at the surface of activated platelets is an essential element for the binding of FVIIIa to the surface of activated platelets. As such, FVIIIa binding to platelet-bound fibrin may promote the assembly of the tenase complex at the platelet surface. Obviously, ACE910/emicizumab lacks this capacity. It is thus possible that the in vivo location of FXa generation in the presence of ACE910/emicizumab is relocalized to areas of increased phosphatidyl-serine exposure.
Regulation of activity. Activity of the FIXa–FVIIIa complex can be downregulated in multiple ways. The natural tenase complex and antibody-driven FXa generation have in common that FIXa is sensitive to inhibition by natural anticoagulants, like antithrombin and protease nexin-2.49,50 However, the tenase complex comprises a second “off” switch, in which FVIIIa cofactor activity is neutralized by following 2 pathways of inactivation, one involving spontaneous dissociation of the A2 domain and a second one involving proteolytic degradation by serine proteases, such as activated protein C. Because tenase activity is limited by its cofactor activity, FVIIIa inactivation is an important means to control FXa generation.
Analysis of ACE910/emicizumab in activity assays
ACE910/emicizumab-dependent enhancement of FXa generation is generally monitored in assay systems that are designed to measure FVIII activity, often using FVIII-deficient plasma. Examples hereof are activated partial thromboplastin time–based clotting assays and tissue factor- or FXIa-induced thrombin generation assays. Because these assays are based on FVIIIa activity being the limited factor, less attention is paid to the amount of FIXa that is generated. However, the extent of FIX activation is different for each of these assays, and it remains difficult to predict how much FIXa would correspond to the amount that is generated in vivo in patients. As mentioned previously, FIXa concentrations determine the activity of ACE910/emicizumab, and consequently, ACE910/emicizumab concentrations equal a different dose of FVIII in each specific assay.51 For instance, in an activated partial thromboplastin time–based assay, 4.0 nM ACE910/emicizumab corresponds 0.1 U/mL FVIII. In tissue factor–based thrombin generation assays, 250 nM ACE910/emicizumab is needed to equal 0.1 U/mL FVIII, whereas 500 nM ACE910/emicizumab is required in FXIa–induced thrombin generation assays to match a 0.1-U/mL FVIII activity. Extrapolation of ACE910/emicizumab cofactor activity values to true FVIII activity concentrations is therefore not possible at this stage of its development. To get some idea about how ACE910/emicizumab compares to FVIII activity units in vivo, one might make use of the annualized bleeding rate that was reported in the HAVEN-1 study (ie, 2.9 events in the group that was assigned to ACE910/emicizumab prophylaxis).16 Although a direct comparison is perhaps premature and incorrect from a methodological point of view, this annualized bleeding rate is remarkably close to the annualized bleeding rate of 2.0 events that has been reported for a group of patients with moderate hemophilia.52 This would suggest that patients under prophylactic treatment with ACE910/emicizumab have a hemostatic potential similar to patients with moderate hemophilia.
Conclusion
The clinical evaluation of ACE910/emicizumab has revealed that the antibody efficiently restores the hemostatic balance in hemophilia A patients with or without inhibitors.15,16 These results have generated unprecedented interest in the community of professionals and patients alike. As researchers in the field of hemophilia, we became aware that some subtleties in how ACE910/emicizumab differs from FVIII in terms of activity were often minimized or not fully appreciated. We therefore decided to perform an objective, fact-based biochemical comparison of the natural cofactor FVIII and the potential therapeutic agent ACE910/emicizumab in order for those using ACE910/emicizumab to have a more complete insight into its mode of action relative to FVIII. We would like to emphasize that in this report, we purposely avoid any judgment on the clinical applicability or quality of the antibody, either positive or negative.
The comparison of both proteins reveals that they are fundamentally different at the various levels. Basically, the only characteristic that emicizumab and FVIIIa have in common is that they both bring FIXa and FX in close proximity. In contrast, in terms of affinity, regulation, topology, and FIXa-enhancing activity, there are important differences. It is thus important to realize that ACE910/emicizumab replaces only part of the full FVIIIa cofactor activity and that the activity of the antibody is predominantly dependent on the amount of FIXa that is generated. This latter characteristic also explains why the activity of emicizumab varies greatly between different activity assays, because the amount of FIXa present in 1-stage clotting assays, 2-stage chromogenic assays, and global thrombin generation assays is substantially different. In addition, it implies that care should be taken when ACE910/emicizumab is used in combination with concentrates that contain FIXa. Monitoring FIXa levels would perhaps be a manner to prevent that uncontrolled hemostasis develops in these patients. Another measure that could be taken to avoid thrombotic complications is to determine the thrombophilic profile of hemophilia A patients (such as FV Leiden status) before combination therapy is applied.
Acknowledgments
The authors thank Roseline D’Oiron (University Hospital Kremlin-Bicetre, France) and Sophie Susen (University Hospital Lille, France) for helpful discussions and James O’Donnell (Royal College of Surgeons, Dublin, Ireland) for correcting English grammar.
Authorship
Contribution: P.J.L., C.V.D., and O.D.C. conceived, wrote, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Lenting, INSERM U1176, 80 rue du General Leclerc, 94276 Le Kremlin-Bicêtre, France; e-mail: peter.lenting@inserm.fr.