Abstract
High-grade B-cell lymphomas (HGBLs) with MYC and BCL2 and/or BCL6 rearrangements, so-called “double-hit” lymphomas (HGBL-DH), are aggressive lymphomas that form a separate provisional entity in the 2016 revised World Health Organization Classification of Lymphoid Tumors. Fluorescence in situ hybridization (FISH) will be required to identify HGBL-DH and will reclassify a subset of diffuse large B-cell lymphomas (DLBCLs) and HGBLs with features intermediate between DLBCL and Burkitt lymphoma into this new category. Identifying patients with HGBL-DH is important because it may change clinical management. This poses a challenge for centers that may not be ready to handle the additional workload and financial burden associated with the increase in requests for FISH testing. Herein, we review the mechanisms of deregulation of these oncogenes. We identify the factors associated with a poor prognosis and those that can guide diagnostic testing. Restricting FISH analysis to the 10% of DLBCL patients who have a germinal center B-cell phenotype and coexpress MYC and BCL2 proteins would be cost-effective and would identify the subset of patients who are at highest risk of experiencing a relapse following conventional therapy. These patients may benefit from intensified chemotherapy regimens or, ideally, should enroll in clinical trials investigating novel regimens.
Introduction
High-grade B-cell lymphomas (HGBLs) represent a spectrum of different diseases. In 2016, the World Health Organization (WHO) revised their classification of lymphoid neoplasms to account for the major advances in lymphoma biology since 2008.1,2 This revision emphasizes molecular features of clinical importance, such as genomic alterations in MYC, BCL2, and/or BCL6 oncogenes. An important change is the addition of HGBL with MYC and BCL2 and/or BCL6 rearrangements, so-called “double-hit” lymphoma (HGBL-DH), as a separate provisional entity.1 This aggressive lymphoma was originally described in 19883,4 and was shown to be associated with a very poor outcome.5 More than 80% of patients with HGBL-DH harbor concurrent translocations in MYC and BCL2.6 The remaining 20% harbor MYC and BCL6 translocations and usually express BCL2 despite not having a BCL2 translocation.7 Two large independent studies have demonstrated that concurrent translocations of MYC and BCL6 are not associated with an inferior outcome in diffuse large B-cell lymphoma (DLBCL).8,9 Thus, MYC and BCL2 oncogenes will be the focus of this review. The coexpression of these proteins in DLBCL, so-called dual expressor DLBCL (DE-DLBCL), has also been associated with an inferior survival, although the outcome is superior to that of HGBL-DH.10-15 DE-DLBCL remains in the DLBCL not otherwise specified (NOS; DLBCL-NOS) category in the revised WHO classification.1
The diagnostic work-up of HGBL-DH is controversial. Although some advocate for extensive cytogenetic testing in all patients with DLBCL to identify the ∼4% of patients who have HGBL-DH, the costs are prohibitive in many centers. A rational approach to fluorescence in situ hybridization (FISH) testing would be useful, especially in institutions with limited resources or access to FISH.
There is also no standard approach to the treatment of HGBL-DH. There is evidence for a superior progression-free survival (PFS) with intensive induction regimens in selected patients.16 However, many HGBL-DH patients are elderly and/or frail, which makes them poor candidates for high-dose chemotherapy. Newer targeted therapies are becoming more readily available, but their role in the treatment of HGBL-DH is not yet established. Herein, we review key concepts regarding MYC and BCL2 deregulation in aggressive lymphomas that may guide diagnostic testing and clinical management.
Biology of MYC and BCL2 in aggressive B-cell lymphomas
MYC protein is detected in 30% to 40% of DLBCLs, 60% of HGBLs, 70% to 100% of Burkitt lymphomas (BLs), and 5% of normal germinal center B (GCB) cells.10,17-19 MYC, located on the long arm of chromosome 8 (8q24), encodes for a transcription factor that, when placed under the transcriptional control of the immunoglobulin (IG) genes, induces aggressive lymphomas in mice.20-22 MYC plays an important role in metabolism, protein synthesis, differentiation, stem-cell renewal, stress response, messenger RNA (mRNA) regulation, and microRNA regulation, as reviewed in Meyer and Penn.23 MYC controls cell cycle progression and proliferation in part by affecting the transcription of cyclin-dependent kinases.24 Paradoxically, induction of MYC can induce genomic instability and apoptosis by increasing the expression of the tumor suppressor TP53 and the proapoptotic protein BIM.25-27 Importantly, MYC is a transcriptional amplifier and increases the transcription of genes that are already poised to be expressed rather than initiating the transcription of new target genes.28,29 Taken together, MYC expression in the context of aggressive lymphomas promotes cell proliferation, induces genomic instability, and amplifies the transcriptional program already in place.
BCL2 protein is detected in >50% of DLBCLs and ∼75% of HGBLs but is not expressed in BL or normal GCB cells.10,18 BCL2 is located on the chromosome arm 18q21 and was originally described in follicular lymphoma (FL).30 BCL2 is a bona fide oncogene,31 but lymphocytes overexpressing BCL2 require additional genomic alterations before developing overt lymphoma.32 The primary function of BCL2 is to promote cell survival by inhibiting apoptosis.33 This is of critical importance in MYC-induced lymphomas, in which genetic instability, DNA damage, and energy stress induce the expression of BIM which, in the absence of BCL2, would bind to effector proteins BAX or BAK and cause mitochondrial depolarization and cell death.27 BCL2 expression not only accelerates the onset of lymphoma growth, it also induces resistance to chemotherapy.34 BCL2 potentiates the effect of other oncogenes and promotes the development of lymphomas in mice that have alterations in EZH2, KMT2D, and GNA13, genes commonly mutated in DLBCL.35-37 BCL2 is one of 6 antiapoptotic proteins (BCL-XL, BCL-W, MCL1, BFL1, and A1) that bind to BH3 proteins in the mitochondria, as reviewed in Letai.38 All of these can accelerate MYC-induced leukemia progression, but BCL2, BCL-XL, and BCL-W are the most potent.39 BCL2 has additional cellular functions in autophagy, mitochondrial energetics, calcium regulation, reduction of reactive oxygen species, and cell cycle regulation, which may also contribute to lymphoma biology.40,41 Overall, BCL2 is an important antiapoptotic protein that synergizes with other oncogenes, especially MYC, to accelerate lymphoma progression.
BCL6 is a transcription factor that orchestrates the germinal center reaction and suppresses MYC and BCL2 expression in normal GCB cells, as reviewed in Basso and Dalla-Favera.42 BCL6 deregulation impairs post-GCB differentiation and induces lymphomas in mice.43 BCL6 protein and BCL6 translocations both contribute to the pathogenesis of DLBCL but are not associated with resistance to rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).8,44
Mechanisms of MYC and BCL2 deregulation in aggressive lymphomas
The main mechanisms of MYC and BCL2 deregulation in aggressive B-cell lymphomas are translocations, mutations, copy number variation, and transcriptional upregulation, mainly by B-cell receptor (BCR) and NF-κB signaling, which vary according to lymphoma subtype (Table 1).
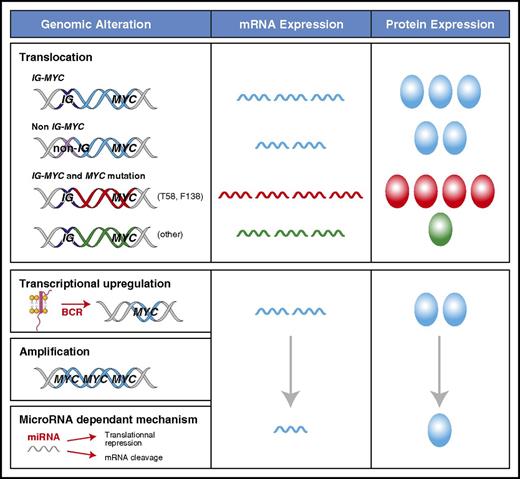
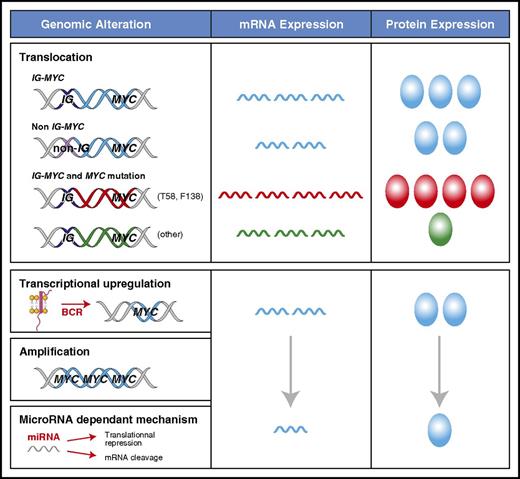
The mechanism of MYC deregulation is important because it can have a dramatic impact on the levels of protein expression (Figure 1). A translocation of MYC to an IG locus leads to the highest levels of MYC mRNA and MYC protein because of the constitutively active transcription driven by the IG promoter.22,45,46 This is present in ∼100% of BLs, 60% of HGBLs, and 5% of DLBCLs.2,9,10,15 In DLBCL, MYC translocations can also occur with a non-IG partner, representing an additional 5% of patients with DLBCL.9 The mechanism of transcriptional deregulation in these non-IG cases is unclear, but the quantity of MYC mRNA and protein is lower than with IG-MYC translocations.9,45,46 MYC can acquire mutations in the context of somatic hypermutation, which can occur in the presence or absence of translocations.47 The prevalence and pattern of MYC mutations in BL (50% to 70%) and DLBCL (5% to 33%) are different.47-49 The gain-of-function MYC mutants (eg, T58 or F138) that are common in BL, occur in <1% of DLBCL patients.47 T58 mutations protect MYC mRNA from degradation, leading to higher levels of MYC protein.50,51 Furthermore, T58 mutants both accelerate cellular proliferation and inhibit apoptosis by decreasing the induction of BIM.27 In contrast, most of the non-T58 or F138 MYC variants, including the polymorphism N11S, decrease MYC protein expression in DLBCL.47 Compared with MYC wild-type, they do not induce cellular proliferation but can still inhibit apoptosis in situations of metabolic stress.47 The MYC gene can also undergo amplification in 8% to 20% of DLBCLs.52,53 However, this has not been consistently shown to be associated with increased MYC protein expression or an inferior clinical outcome.17,53-58 Other factors can affect MYC levels without directly altering the integrity or location of the MYC locus. Many signaling pathways, including BCR and NF-κB signaling, can lead to increased transcription of MYC through direct and indirect interactions at the MYC promoter as reviewed by Wierstra and Alves.24
Mechanisms of MYC deregulation in aggressive lymphomas. The levels of MYC mRNA expression and protein can vary according to the mechanism that is driving transcription and the presence of MYC mutations, which are reflected as different colors for mRNA and protein (blue for wild type). MYC mRNA is the highest in the context of an IG-MYC translocation. Decreased mRNA degradation in patients who have MYC T58 mutations (red) can further increase MYC mRNA levels, whereas other mutations can decrease MYC protein levels (green). MYC transcription can be increased through mechanisms other than translocations, but they are more variable and generally result in lower MYC protein expression.
Mechanisms of MYC deregulation in aggressive lymphomas. The levels of MYC mRNA expression and protein can vary according to the mechanism that is driving transcription and the presence of MYC mutations, which are reflected as different colors for mRNA and protein (blue for wild type). MYC mRNA is the highest in the context of an IG-MYC translocation. Decreased mRNA degradation in patients who have MYC T58 mutations (red) can further increase MYC mRNA levels, whereas other mutations can decrease MYC protein levels (green). MYC transcription can be increased through mechanisms other than translocations, but they are more variable and generally result in lower MYC protein expression.
The mechanisms of deregulation for BCL2 are similar to those of MYC, although BCL2 translocations are usually early events that occur as a result of aberrant variable diversity joining recombination of the IG gene, mediated by RAG1/2 in the bone marrow.59 BCL2 is mutated in 68% of GCB-DLBCL and 6% of activated B-cell (ABC) –DLBCL.60 BCL2 mutations are associated with the presence of a BCL2 translocation and BCL2 protein expression.60 They consistently spare the BH3 domain, which is necessary for its antiapoptotic function and is the binding site for BH3 mimetics such as venetoclax.60,61
Biology of HGBL-DH and DE-DLBCL
The pathogenesis of HGBL-DH involving MYC and BCL2 oncogenes can be characterized by a temporal sequence of events that begin with the BCL2 translocation t(14;18) as the first hit, followed by a subsequent hit with the acquisition of the MYC translocation in the germinal center, either as an early or late event in the disease.3,6 HGBL-DHs have a GCB phenotype in 90% of patients, but the expanded definition of HGBL-DH to include dual translocations in MYC and BCL6 will group more patients with an ABC phenotype into this category. Overall, 60% of patients with HGBL-DH have concurrent MYC and BCL2 translocations, 20% implicate MYC and BCL6 only, and 20% have breakpoints in all 3 oncogenes (ie, triple-hit lymphoma).6,8 Although the numbers in each study are small,8-10,62,63 it seems that ∼20% of patients with HGBL-DH do not express either MYC or BCL2 proteins despite harboring the gene translocations. This is clinically relevant because the outcome of patients who have HGBL-DH without MYC or BCL2 protein expression is likely more favorable than the outcome for those who have both HGBL-DH and are dual expressors.10,62,63 The lack of protein expression may be explained by further genomic events such as insertions, gene rearrangements near or within the MYC or BCL2 translocation sites, MYC or BCL2 gene mutations, and translocation of MYC to a non-IG partner gene.9,47,62,64,65 Finally, HGBL-DHs have complex karyotypes and additional genomic events that have not yet been fully characterized.6,62 TP53 mutations are present in 20% to 30% of patients, which can further induce genomic instability and contribute to therapeutic resistance.66,67
The coexpression of MYC and BCL2 proteins in DLBCL (ie, DE-DLBCL), does not define a specific tumor biology, but rather it should be considered a prognostic biomarker of a poor outcome. This has been a consistent finding across 8 independent studies8,10-15,68 (Table 2), supporting preclinical data showing that the coexpression of these proteins is pathogenic. Remarkably, those data have been reproducible despite using different antibody clones and thresholds.19,69 Furthermore, coexpression of these proteins is prognostic at diagnosis and relapse. Beyond R-CHOP, it predicts for a poor response to intensified induction regimens, salvage chemotherapy, autologous stem cell transplant (ASCT), and the novel histone deacetylase inhibitor panobinostat.63,68,70,71 BCL2 and MYC protein expression in DE-DLBCL may be considered a functional readout of the cumulative genomic alterations that ultimately lead to their overexpression, mainly BCR and NF-kB signaling in the ABC patients and translocations in the GCB patients. These mechanisms may be clinically relevant, given that targeting BCR signaling and BCL2 may be an effective strategy in ABC-DE-DLBCL but not in GCB-DE-DLBCL or in patients with IG translocations.72
One contentious issue has been the prognostic significance of an isolated MYC translocation in DLBCL in the absence of a BCL2 translocation. Unfortunately, 60% to 80% of these patients still express BCL2 protein through other mechanisms, and determining a difference in outcome on the basis of BCL2 expression is challenging because of the limited number of these patients in each study.9,73-75 In a large cohort of patients with DLBCL, the outcome of those with an MYC translocation but without BCL2 expression was excellent, with 90% remaining in remission beyond 5 years.10 Thus, the poor prognosis associated with IG-MYC translocations is driven mainly by the high DE-DLBCL patients, a situation that may be similar for those with HGBL-DH.9
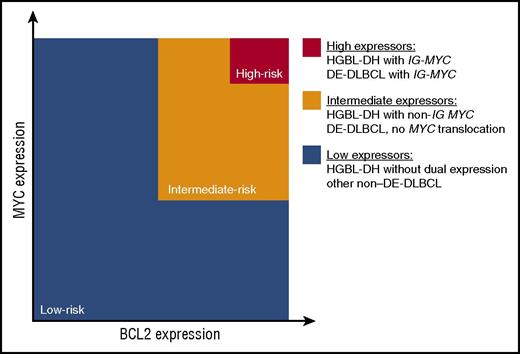
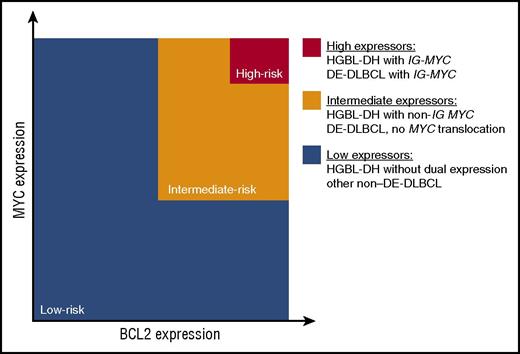
Overall, the body of literature addressing MYC and BCL2 expression in DLBCL is consistent with the model highlighted in Figure 2. DE-DLBCL represents many diseases that deregulate MYC and BCL2 through a variety of mechanisms, but their unifying feature is that they are associated with a poor outcome. Within this group, the subset of HGBL-DH and DE-DLBCL with IG-MYC translocations that express high levels of MYC and BCL2 proteins seem to have the worst outcome. In contrast, HGBL-DH is genetically more homogeneous: transcription of MYC and BCL2 is under the control of another gene. The heterogeneity in clinical outcome is a result of ongoing genetic events that decrease MYC and BCL2 expression, non-IG MYC partners, and the presence of TP53 mutations.
Model assessing clinical risk according to MYC and BCL2 status in DLBCL. The body of literature supports a model whereby the risk of treatment failure is proportional to the degree of MYC and BCL2 protein expression, which in turn is determined by the mechanism of deregulation. Co-expression of MYC and BCL2 (in orange and red) occurs in 25% to 30% of patients. The 5% of patients with the worst clinical outcome (in red) have the highest expression of MYC generated from a translocation to the IG locus. The intermediate-risk patients (in orange) include those with HGBL-DH that express lower levels of MYC protein due to a non-IG MYC translocation and the DE-DLBCL that deregulate MYC and BCL2 from other mechanisms. The low-risk category (in blue) consists of all patients with non-DE-DLBCL, including HGBL-DH that are not dual expressors and DLBCL with a MYC translocation, but without BCL2 protein expression. Note that HGBL-DH has MYC translocations and BCL2 or BCL6 translocations, and DE-DLBCL expresses both MYC and BCL2 proteins.
Model assessing clinical risk according to MYC and BCL2 status in DLBCL. The body of literature supports a model whereby the risk of treatment failure is proportional to the degree of MYC and BCL2 protein expression, which in turn is determined by the mechanism of deregulation. Co-expression of MYC and BCL2 (in orange and red) occurs in 25% to 30% of patients. The 5% of patients with the worst clinical outcome (in red) have the highest expression of MYC generated from a translocation to the IG locus. The intermediate-risk patients (in orange) include those with HGBL-DH that express lower levels of MYC protein due to a non-IG MYC translocation and the DE-DLBCL that deregulate MYC and BCL2 from other mechanisms. The low-risk category (in blue) consists of all patients with non-DE-DLBCL, including HGBL-DH that are not dual expressors and DLBCL with a MYC translocation, but without BCL2 protein expression. Note that HGBL-DH has MYC translocations and BCL2 or BCL6 translocations, and DE-DLBCL expresses both MYC and BCL2 proteins.
Clinical presentation
The histologic diagnosis of double-hit lymphomas has changed considerably over the years. More than 95% of patients present with DLBCL or with a high-grade lymphoma that shares features of DLBCL and BL, formerly categorized as B-cell lymphoma unclassifiable but which has been reclassified as HGBL-NOS. Both can occur at the time of diagnosis or following histologic transformation from an indolent lymphoma, but this distinction does not have prognostic significance.62,76 Rarely, they can also present as acute lymphoblastic lymphoma or FL. A high-grade morphology is more commonly associated with an IG-MYC translocation, disseminated disease, and a worse outcome, although this is not consistent across studies.16,62
More than 90% of patients with HGBL-DH present with high-risk clinical features defined as having one of the following: leukocytosis, central nervous system (CNS) involvement, lactose dehydrogenase greater than 3 times the upper limit of normal, and advanced-stage disease.16,62 Routine FISH testing in all patients with HGBLs (including DLBCL) has led to an increased number of patients identified as low-risk HGBL-DH (ie, lacking the high-risk features listed above).77 These patients would never have been subjected to cytogenetic analysis in the past and now seem to have a better clinical outcome, with the caveat that many of these low-risk patients are currently treated with more intensive therapies.16 Patients with DE-DLBCL, but without MYC and BCL2 translocations, can also present with aggressive clinical features and an increased International Prognostic Index. Identifying these patients may be clinically important because they have a 10% risk of developing a CNS relapse at 2 years.78 Thus, a thorough evaluation of the cerebrospinal fluid at the time of diagnosis is indicated for patients with either HGBL-DH or DE-DLBCL.16,78,79
Rational FISH testing in aggressive B-cell lymphomas
Given that HGBL-DH is its own provisional entity and that this diagnosis may change clinical management, an increase in FISH testing is expected. FISH testing is indicated in patients in whom BL is a consideration and in those who have a >20% incidence of MYC translocations, such as HGBL-NOS, plasmablastic lymphoma, and patients with histologic transformation from antecedent FL.18,19,77 To truly rule out HGBL-DH in DLBCL, FISH would be required in all patients, even though its prevalence is very low. The most reliable technique is interphase FISH with break-apart probes that would also detect non-IG MYC translocations. To further define the translocation partner, additional tests using fusion probes spanning MYC and IGH, IGκ, or IGλ could be performed but are not required for the diagnosis.
Immunohistochemistry can be useful for screening patients who require FISH because it is inexpensive, accessible, and routinely used in clinical laboratories.19 The proliferation marker Ki-67 is always increased in BL but is more variable in HGBL-DH and is not a reliable marker for screening patients that require FISH.10 Factors affecting poor concordance between hematopathologists include crush artifact and variation in staining intensity and tumor content.80 Furthermore, thresholds that categorize patients as being positive vary among studies and still require prospective validation. Although BCL2 expression is routinely assessed in pathology, MYC expression is not assessed in all laboratories. The MYC antibody clone Y69 has good interobserver reproducibility.10,58,81 The 40% threshold for MYC and 50% threshold for BCL2 were initially selected on the basis of their prognostic value, not as thresholds for screening patients that require FISH.10 It is reasonable to continue using these thresholds until a consensus is reached, but reporting the percentage of positive cells in the biopsy would be ideal because extremes in protein expression are more reproducible and are helpful in assessing the likelihood of detecting an MYC translocation.9,10,58
In centers where FISH analysis is not feasible for all patients with DLBCL, using cell of origin with MYC and BCL2 coexpression would be a useful screening strategy to test patients who are at highest risk of treatment failure. Restricting FISH testing to patients with a GCB phenotype would reduce the number of patients to test by ∼50%. However, on the basis of data from more than 1000 patients included in 2 independent studies,8,10 only 6% of GCB patients will have HGBL-DH (Table 1). Selecting GCB DLBCLs that also express MYC and BCL2 proteins would reduce testing by >90% because only 15% of patients with GCB-DLBCL will also be dual expressors. By using this strategy, one-third of DLBCLs probed by FISH would be a bona fide HGBL-DH. The HGBL-DH missed would be enriched in cases harboring MYC and BCL6 translocations and those that do not have dual expression, whose clinical significance is unclear.
Treatment
The optimal induction regimens for HGBL-DH and DE-DLBCL have not yet been established. The outcome of HGBL-DH has historically been poor with R-CHOP; thus, more intensive induction regimens have been evaluated in young and fit patients.16 The retrospective study by Petrich et al16 included the largest series of patients with HGBL-DH to date, and different induction regimens were evaluated in 311 patients. More intensive regimens were associated with a PFS of 22 months compared with 8 months with R-CHOP, but there was no difference in overall survival. Dose-adjusted rituximab plus etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH) had a superior complete response rate compared with R-CHOP and was less toxic than other high-dose regimens; thus, it has been adopted as one of the preferred induction regimens for HGBL-DH in many centers.16,82 In this study, 72% of patients had a good performance status, indicative of patients being selected to exclude those who were frail.16 Furthermore, over half of the patients included in the study were treated after 2009, which reflects that increased cytogenetic testing in the modern era resulted in an increased detection of HGBL-DH with low-risk features. Prospective and unselected cytogenetic testing of all high-grade lymphomas at a single institution recently identified many patients with low-risk HGBL-DH who were cured with R-CHOP alone.77 This is in contrast to selected high-risk patients in early reports who were not cured with intensive regimens and/or ASCT.5,62,83-87 No study has yet demonstrated an advantage to using an intensive induction regimen for patients with DE-DLBCL. MYC and BCL2 coexpression was associated with a poor outcome in DLBCL patients treated in clinical trials that investigated the efficacy of ASCT after either R-CHOP or dose-intensified R-CHOP.68,88 Investigating for the presence of CNS involvement is indicated, given its high prevalence in HGBL-DH and DE-DLBCL. CNS prophylaxis may be beneficial, but there are insufficient data to support that systemic CNS prophylaxis is superior to intrathecal chemotherapy.16
The role of ASCT in HGBL-DH and DE-DLBCL is controversial. For HGBL-DH patients in complete remission after induction therapy, consolidation with ASCT was associated with a trend toward improved overall survival in the Petrich study.16 However, there is an inherent selection bias when offering transplantation to patients who have fewer comorbidities and chemotherapy-sensitive disease.16 In the Southwest Oncology Group (SWOG) S9704 study, there was a trend toward an improvement in PFS for DE-DLBCL patients receiving a transplant, but this was not statistically significant, given that data were available in only 12 patients.86 The 4 patients with HGBL-DH included in that study died of progressive lymphoma.86 At the time of relapse, the outcome of patients with HGBL-DH and DE-DLBCL is extremely poor. In the Collaborative Trial in Relapsed Aggressive Lymphomas (CORAL), MYC translocations predicted for a poor response to salvage chemotherapy and ASCT in patients with relapsed or refractory DLBCL (rrDLBCL), in which the 3-year PFS was only 17% to 19%.75 BCL2 protein was expressed in 81% of those rrDLBCLs.75 Similarly, none of the patients with rrDE-DLBCL achieved a durable remission with salvage chemotherapy and ASCT in the Canadian LY12 (CAN-NCIC-LY12) study.70,89 The 3-year event-free survival for the patients with rrDE-DLBCL was 0% vs 61% for the patients who did not have DE-DLBCL (P < .001).70 Herrera et al63 studied the prognostic impact of dual expression and HGBL-DH in 117 patients who had initially responded to salvage chemotherapy and had successfully undergone ASCT for rrDLBCL. Although DE-DLBCL and HGBL-DH were each associated with an inferior outcome, patients with concurrent HGBL-DH and DE-DLBCL all experienced an early lymphoma relapse within months of the transplant.63
Approximately 20% to 30% of patients with HGBL-DH have primary refractory disease, despite receiving intensive induction regimens, which indicates an unmet clinical need to improve the initial treatment strategy in this disease.16 Enrollment in a clinical trial testing regimens that include novel therapies would be the preferred choice for eligible patients. Targeting BCL2 protein is a logical approach because R-CHOP is curative in >90% of patients with DLBCL who harbor isolated MYC translocations without BCL2 protein expression or translocations.10,90 Furthermore, silencing BCL2 expression resulted in tumor regression in mouse models of lymphoma that were positive for both MYC and BCL2.91 The BH3 mimetic venetoclax, formally known as ABT-199, is a BCL2-specific inhibitor that has recently been approved by the US Food and Drug Administration for the treatment of chronic lymphocytic leukemia.61,92,93 Venetoclax synergized with cyclophosphamide or bendamustine and rituximab and cured mice transplanted with murine or human HGBL-DH.61 Early results from the phase 1/2 trial investigating the safety and efficacy of venetoclax and CHOP, in combination with either rituximab or obinutuzumab, have recently been reported in abstract form.94 Although the preliminary results seem promising with an overall response rate of 91%, the only 2 patients (2 of 13) who had progressive disease in this trial had DE-DLBCL. Thus, inhibiting BCL2 alone may not be sufficient in some patients, and this first-line resistance to venetoclax deserves further investigation. For instance, targeting additional antiapoptotic proteins known to cause resistance to venetoclax (eg, MCL1 or BCL-XL) may be required.95,96 Targeting MYC may be helpful, but identifying specific inhibitors to MYC protein has been an ongoing challenge, as reviewed in McKeown and Bradner.97 MYC has a shorter half-life than BCL2 (∼4 vs ∼24 hours), and inhibiting translation or transcription is effective in treating Eμ-Myc lymphomas.97,98 In fact, this may be one of the mechanisms by which conventional chemotherapy, such as doxorubicin, can decrease MYC protein levels,99 and why it is effective in curing human BL.100,101
The revision of the WHO classification coincides with a time when there are several targeted therapies that are approved by the Food and Drug Administration for other indications and could theoretically be prescribed off label to treat patients with HGBL-DH or DE-DLBCL. Supporting this claim is a recent case report using the immunomodulating agent lenalidomide to extend the duration of response in a patient with HGBL-DH who had a CNS relapse.102 The BTK inhibitor ibrutinib can decrease levels of MYC protein in primary chronic lymphocytic leukemia cells exposed to IgM antibodies that stimulated BCR signaling.103 In DLBCL, the best responses to ibrutinib occurred in patients with the ABC subtype, which can generate MYC and BCL2 from BCR signaling.104 Bortezomib has been shown to decrease cell proliferation in BL cell lines, in part by decreasing expression of MYC protein.105 Ibrutinib and bortezomib synergize with venetoclax in DLBCL cell lines and a mouse model of HGBL-DH.92,106 The combination of novel drugs, however, is not advised outside the context of clinical trials, given the potential for serious toxicities, including tumor lysis syndrome known to be associated with venetoclax.107,108 Lenalidomide, ibrutinib, and bortezomib have been safely combined with R-CHOP, and it would be helpful to analyze the tumor biopsies from the patients treated with these regimens to determine whether MYC and BCL2 coexpression or translocations are biomarkers of response.109-112 There are also clinical trials investigating novel agents that may decrease MYC and/or BCL2 protein expression or inhibit their function, but this is beyond the scope of this review. Testing the efficacy and safety of these novel agents, in combination with chemotherapy, is the most promising step toward finding better induction and salvage regimens for patients with HGBL-DH and DE-DLBCL.
Conclusion
BCL2 rescues lymphoma cells from MYC-induced apoptosis. The combination of MYC and BCL2 is synergistic. Together they accelerate lymphoma progression and induce resistance to chemotherapy. Multiple studies involving patients with DLBCL confirm that dual expression is associated with poor survival. The mechanism of MYC deregulation can affect the levels of protein expression, with the highest levels seen in IG-MYC translocations. This dosage effect may explain why HGBL-DH and DE-DLBCL harboring an IG-MYC translocation seem to have the worst clinical outcome. Low MYC protein expression is associated with a favorable outcome and, in the context of an MYC translocation, can occur as a consequence of MYC mutations or a non-IG MYC partner gene. FISH testing is required for the diagnosis of HGBL-DH. Selecting only patients with DLBCL with GCB and dual expression would reduce FISH testing by >90%. Data supporting the use of more intensive treatment regimens such as dose-adjusted R-EPOCH to improve PFS have been in the context of HGBL-DH. Clearly, new therapies are required for the substantial proportion of patients with HGBL-DH or DE-DLBCL who have primary refractory disease. Establishing the correct diagnosis is the first step in identifying these high-risk patients so they can be enrolled in the appropriate clinical trials investigating the combinations of agents that directly or indirectly target MYC and/or BCL2.
Acknowledgments
The authors are grateful to colleagues who have provided helpful feedback for this review. In particular, the authors thank Martin Dyer, Randy Gascoyne, Sarit Assouline, and Martin Gyger for their helpful comments, which have significantly improved the manuscript.
This study was supported by operating Grant No. 299607 from the Canadian Institute for Health Research (N.A.J.), Innovation Grant No. 703425 from the Canadian Cancer Society Research Institute, and the Jewish General Hospital Foundation. N.A.J. received funding from le Fond de Recherche en Santé du Québec.
Authorship
Contribution: P.S. and N.A.J. researched data for the article, wrote the manuscript, and reviewed the article before submission.
Conflict-of-interest disclosure: N.A.J. received research funding from Roche Canada and AbbVie and consulting fees and/or honoraria from AbbVie, Roche, Lundbeck, Janssen Pharmaceuticals, and Gilead Sciences. P.S. declares no competing financial interests.
Correspondence: Nathalie A. Johnson, Departments of Medicine and Oncology, Jewish General Hospital, 3755, chemin de la Côte-Ste-Catherine, Montréal, QC H3T 1E2, Canada; e-mail: nathalie.johnson@mcgill.ca.