In this issue of Blood, Gorini et al provide compelling evidence for an indirect CD1d- and natural killer T (NKT) cell–dependent mechanism that promotes disease progression in chronic lymphocytic leukemia (CLL).1
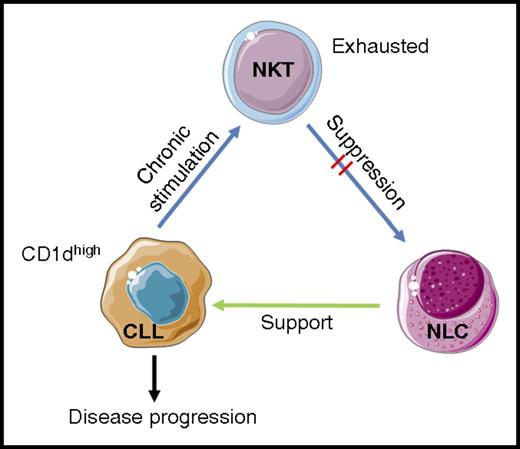
Model for CD1d-dependent CLL disease progression. Gorini and colleagues postulate that chronic stimulation of NKT cells with high levels of CD1d/self-ligand complexes on CD1dhi CLL cells leads to NKT cellular exhaustion. The exhausted NKT cells therefore have a diminished ability to engage and suppress differentiation and function of CD1d+ NLCs. Removal of NLC suppression allows them to support CLL cells, thus increasing the chance of disease progression. Images adapted from Servier Medical Art under a Creative Commons CC-BY license.
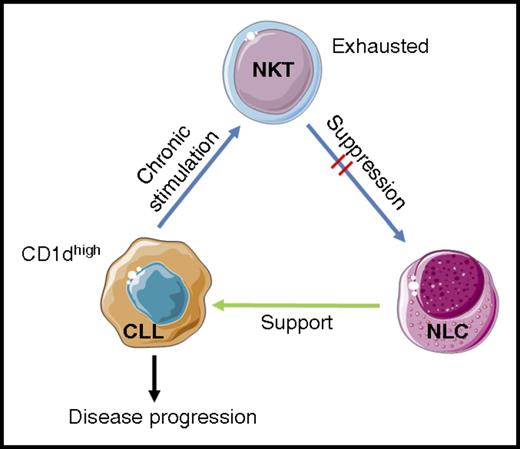
Model for CD1d-dependent CLL disease progression. Gorini and colleagues postulate that chronic stimulation of NKT cells with high levels of CD1d/self-ligand complexes on CD1dhi CLL cells leads to NKT cellular exhaustion. The exhausted NKT cells therefore have a diminished ability to engage and suppress differentiation and function of CD1d+ NLCs. Removal of NLC suppression allows them to support CLL cells, thus increasing the chance of disease progression. Images adapted from Servier Medical Art under a Creative Commons CC-BY license.
CLL is the most prevalent adult leukemia, characterized by expansion of CD5+ B cells. Although some patients maintain stable disease, others display a more aggressive phenotype with disease progression. Over the past 5 to 6 years, a murky relationship between CLL CD1d expression, numbers and activation status of CD1d-restricted NKT cells, and disease progression has started to come into focus.
CD1d expression has promise as a diagnostic marker for CLL.2 Using sizeable cohorts, low CD1d expression has been demonstrated in CLL cells compared with normal B cells from the same patients. Low CD1d expression may also be a good diagnostic marker for CLL because it is not observed in significant numbers of patients with other hematological malignancies. However, other groups have observed patients with CLL displaying high levels of CD1d expression, and high CD1d expression has been correlated with a poor prognosis.3,4 Furthermore, as in several cancers, NKT frequencies and total numbers appear to be diminished in patients with progressive CLL compared with patients with stable disease or with healthy controls.5
In the study by Gorini et al, the authors have astutely hypothesized that the relationship between CD1d+ CLL cells and T-cell antigen receptor–bearing NKT cells is not as simple as a 2-way interaction leading to tumor control or killing. The authors used the Tcl1 transgenic mouse model of CLL, which spontaneously develops CD19+CD5+IgM+B220loCD1d+ B lineage CLL cells. By backcrossing onto mice lacking type I or type I and II NKT cells, a faster and more aggressive tumor progression was achieved than in Tcl1 controls. This was unexpected because type II NKT cells are thought to counter type I NKT cell antitumor responses. Further study will be required to determine if type II NKT cells uniquely contribute to immunosurveillance in CLL, or if the antitumor effects of the type I NKT cells outweigh that of the type II NKT cells in the Tcl1 model. By using adoptive transfer approaches, the team also demonstrated that CD1d expression by CLL cells was not required for initial tumor control, implicating a third player in CD1d-dependent tumor surveillance by NKT cells.
Gorini et al then explored these findings with a series of experiments using samples from patients with stable and progressive CLL, confirming previous reports that high CD1d expression was more evident in the group with progressive CLL. NKT cell function was impaired in patients with progressive CLL, with poor responsiveness to stimulation with strong agonists, such as CD3/CD28 or phorbol myristic acid/ionomycin. This indicated that strong CD1d stimulation rendered NKT cells unresponsive to additional stimulation. Interestingly, these effects were observed in the absence of any exogenous CD1d ligand, demonstrating that a CLL self-ligand could be driving NKT cell exhaustion. Although the nuances of NKT cell anergy versus exhaustion were not explored due to a lack of material, these observations suggest that additional studies could be done to functionally characterize the “exhausted” NKT cells. Such characterization might also involve sublineage determination and whether there is any skewing away from an antitumor Th1 phenotype.
The authors then tested the hypothesis that NKT cells could suppress the CLL-supporting functions of monocyte-derived CD1d+ nurse-like cells (NLCs). T cell–depleted peripheral blood mononuclear cells (containing CD1d+ monocytes and CD1dlo or CD1dhi CLL cells) were cultured with healthy donor NKT cells. It was demonstrated that under conditions of low CD1d expression by CLL cells, NLCs did not differentiate and CLL cells were reduced in number. In contrast, cultures containing CD1dhi CLL cells resulted in higher numbers of differentiated NLCs and CLL cells.
Previously, one would understandably hypothesize that loss of CD1d expression by CLL cells could lead to evasion of NKT cell control. As a result of the new studies by Gorini and colleagues, a model was developed in which CLL evades NKT control indirectly. Under conditions of low CD1d expression by CLL cells, NKT cells are able to control the tumor via interaction with CD1d-expressing monocytes and NLCs, limiting their differentiation and ability to support CLL cells. However, under conditions of high CD1d expression, overstimulation (or at least chronic stimulation) leads to NKT cell exhaustion, such that they are unable to suppress NLC differentiation and subsequently support NLCs (see figure).
Although investigators and clinicians will await independent confirmation of these findings, this intriguing new model may have far-reaching implications for CLL diagnosis and treatment and may also impact research outside this immediate field. Firstly, CLL may remain a very useful diagnostic marker for early-stage and stable CLL. Furthermore, the use of exogenous CD1d-binding glycolipids to boost NKT cell control of CLL, as suggested in previous studies,3 could be problematic. Under certain conditions, CD1d-binding glycolipids, such as α-galactosylceramide, render NKT cells anergic.6 If such glycolipids are used for therapy, they could lead to desuppression of NLCs and have the unintended consequence of leading to more aggressive CLL.
A functional link between CD1d expression by B-lineage cells and NKT cells is also contributory to the humoral immune response to vaccination,7 to blood-group antigen alloreactivity,8 and to NKT cell–regulated autoreactive B cells in systemic lupus erythematosus.9 Investigators in these other fields may wish to consider the consequences of low versus high CD1d expression by B-lineage cells and indirect mechanisms involving other CD1d+ cell types.
The study by Gorini et al therefore sheds important new light on the mechanisms underlying the progression of CLL and may impact other areas of study. Arguably, new therapeutics for CLL may involve breaking the cycle between CD1dhi CLL cells, NKT cells, and NLCs.
Conflict-of-interest disclosure: The author declares no competing financial interests.